A lupus anticoagulant detection reagent
A lupus anticoagulant and detection reagent technology, which is applied in the field of clinical detection, can solve problems such as difficult matching, increased false positive rate or false negative rate of test results, etc., and achieves the effects of easy production, improved accuracy, and improved sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
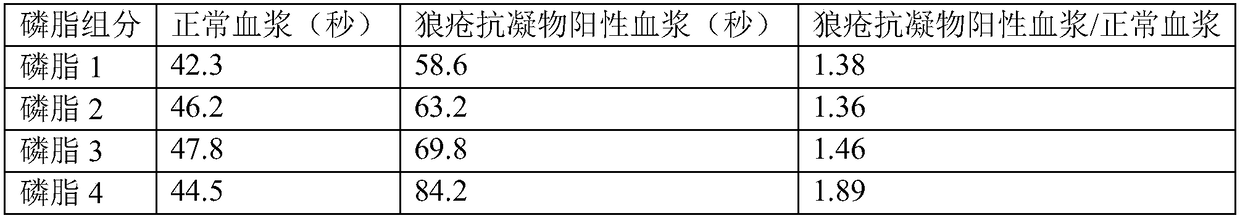
[0024] The screening of embodiment 1 synthetic phospholipids
[0025] Weigh an appropriate amount of synthetic phosphatidylserine, phosphatidylcholine, phosphatidylglycerol, phosphatidylethanolamine, and cholesterol, and dissolve them in chloroform in different proportions (accounting for the mass percentage of synthetic phospholipids) (see Table 1 for details), mix well, and use Blow dry with nitrogen, then vacuum to dry residual chloroform. Add a certain amount of distilled water and stir at room temperature for 2 hours to prepare a phospholipid liposome solution. Store at -20°C for later use.
[0026] Table 1
[0027] Phospholipid component
10%
30%
40%
15%
5%
10%
35%
35%
15%
5%
Phospholipid 3
10%
45
35%
10%
5%
Phospholipid 4
5%
30%
40%
...
Embodiment 2
[0035] Embodiment 2 The preparation of the reagent of lupus anticoagulant of the present invention
[0036] (1) Preparation of screening reagents
[0037] Prepare 50mM Tris buffer, add 100mM sodium chloride, add appropriate amount of silica (final concentration is 0.28%), add stabilizers and preservatives, so that the solution contains 1% BSA by mass volume, 1% PEG6000, 0.02% BHT , 0.05% sodium azide, adjust the pH to 7.35, add an appropriate amount of the prepared phospholipid 4 solution in Example 1, so that the final concentrations are 10 μM, 40 μM, 160 μM and mix well. Set up the program according to the instructions of the instrument, and use the prepared screening reagent to test the normal plasma and lupus anticoagulant positive plasma on the Sysmex CA1500 hemagglutination analyzer. The test parameters are: take 50 μl of the screening reagent and add it to the reaction tube. , add 50 μl of blood sample, incubate at 37°C for 3 minutes, add 50 μl of calcium chloride solu...
Embodiment 3
[0046] Embodiment 3 Stability
[0047] Prepare screening reagents and diagnostic reagents according to Example 2, place the reagents at 4°C, incubate at 37°C, and take samples for detection every 7 days. The results are shown in Table 5.
[0048] table 5
[0049]
[0050] The results showed that the detection results of the lupus inhibitor detection reagent did not change much after being stored at 37°C for 28 days, and the stability was very good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com