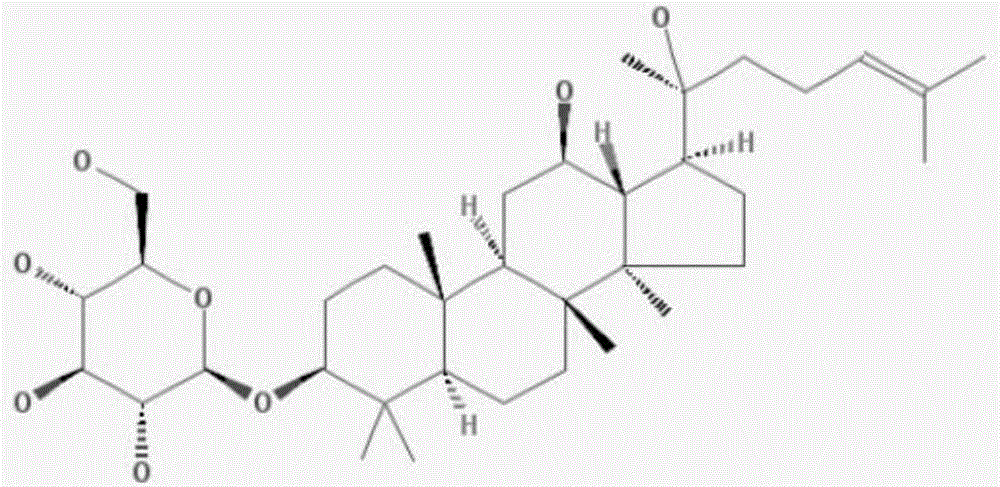
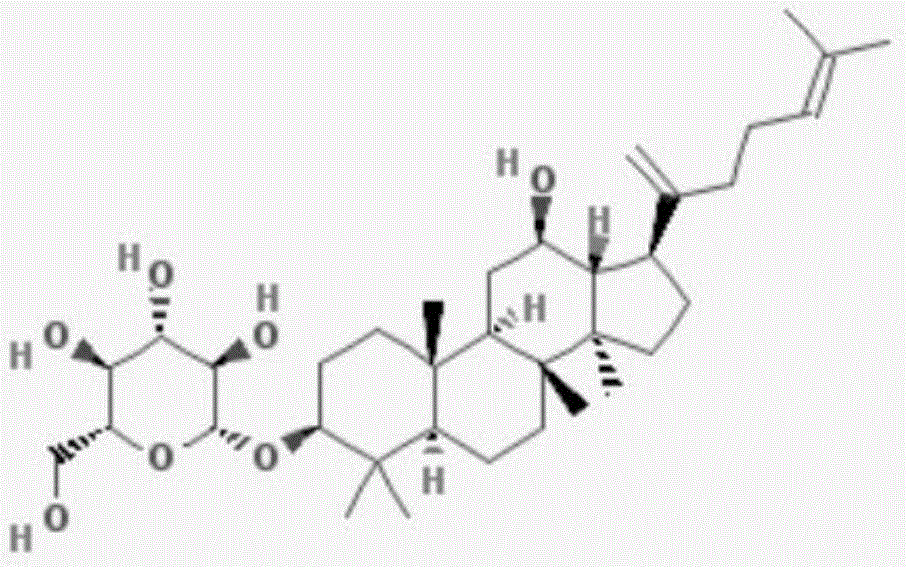
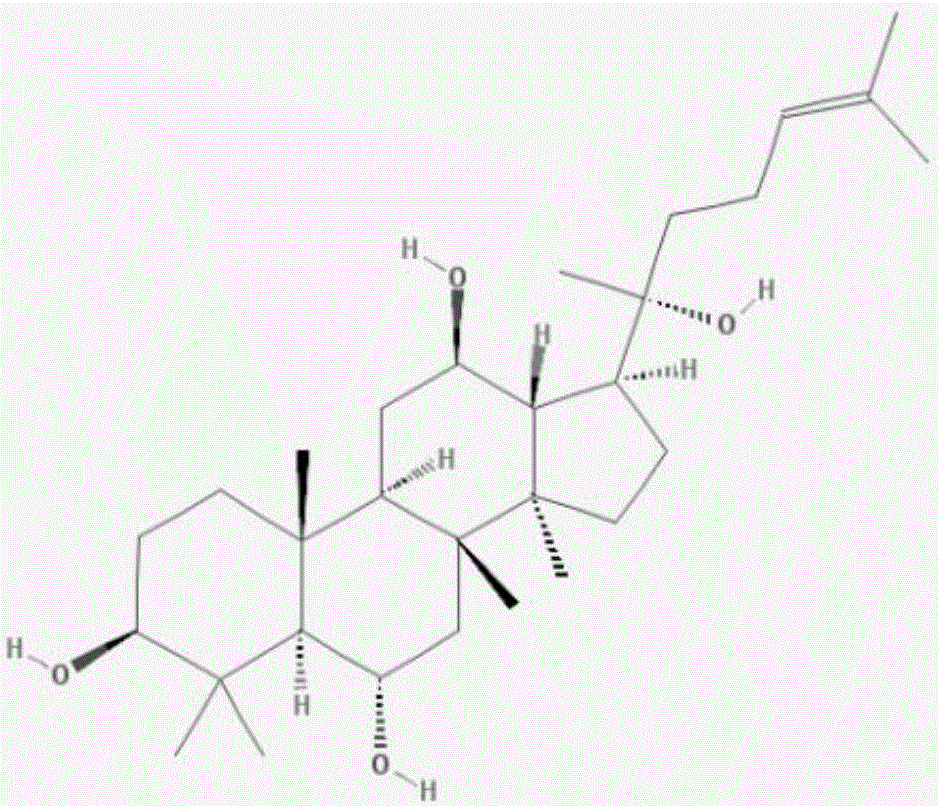
Application of ginsenoside in preparing antiphospholipid syndrome molecular targeting treatment medicine
A technology of molecular targeted therapy and ginsenosides, which is applied in drug combinations, medical preparations containing active ingredients, and pharmaceutical formulas, can solve the problems of high recurrence rate of antiphospholipid antibody syndrome
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Safety Evaluation
[0032] The research results of the non-clinical safety evaluation of the present invention are as follows:
[0033] 1. Oral acute toxicity test in mice
[0034] Under the conditions of maximum dosage concentration and maximum dosage volume, mice were orally given (20S)-ginsenoside Rh212g / kg by gavage, and observed continuously for 14 days, no death or abnormal toxic reaction was found in the animals. Show that (20S)-ginsenoside Rh2 mice gavage maximum tolerated dose is 12g / kg.
[0035] 2. Beagle dog oral acute toxicity test
[0036] Under the conditions of the maximum dosage concentration and maximum dosage volume, Beagle dogs were given (20S)-ginsenoside Rh2 2g / kg orally by gavage, and observed continuously for 14 days. No death or abnormal toxic reaction was found in the animals. It shows that (20S)-ginsenoside Rh2Beagle canine gavage maximum tolerated dose is 2g / kg.
[0037] 3. Long-term toxicity of intragastric administration in rats ...
Embodiment 2
[0045] Embodiment 2 pharmacodynamic evaluation
[0046] Research result of the present invention is as follows:
[0047] 1. (20S)-ginsenoside Rh2 binds Annexin A2 and weakens its interaction with β 2 -GP1 interaction
[0048] Materials: recombinant human Annexin A2 protein, recombinant human β 2 -GP1 expressed in Escherichia coli and purified by high performance liquid chromatography; (20S)-ginsenoside Rh2 (China National Institutes for Food and Drug Control, purity: 99.0%, batch number: 111748).
[0049] method
[0050] 1.1. Dilute 100 μg recombinant human Annexin A2 protein and 10 μg (20S)-ginsenoside Rh2 in 1.5 mL phosphate buffer, without adding (20S)-ginsenoside Rh2 to the control group, incubate at 37°C for 1 hour, and dispense 100 μl / tube to 12 tubes, heat 12 tubes at different temperatures (heating temperatures are 40, 43, 46, 49, 52, 55, 58, 61, 64, 67, 70, 73°C) for 3 minutes, centrifuge at 20000×g for 20 minutes, SDS polyacrylamide gel electrophoresis detects t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com