Kit for removing rivaroxaban in plasma in vitro
A rivaroxaban and kit technology, applied in the field of biomedicine, can solve the problems of large thrombosis risk, test results misleading clinical diagnosis and treatment, false positives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
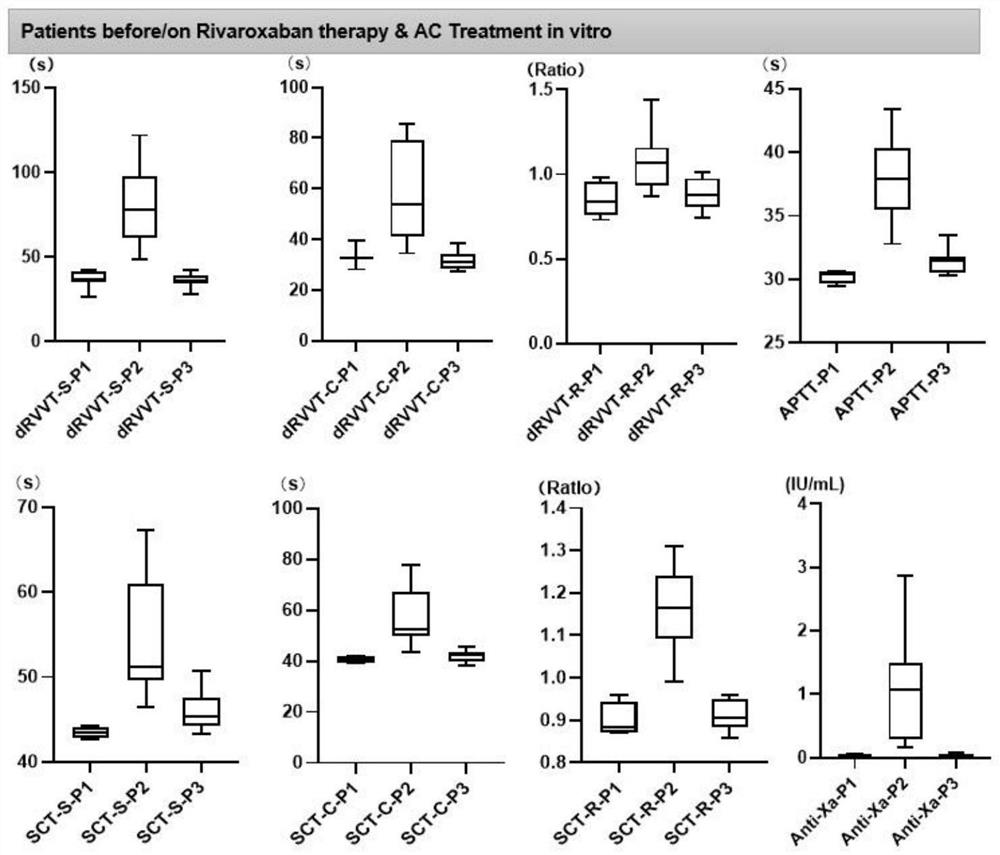
[0021] The present invention provides a kit for removing rivaroxaban from blood plasma. The kit includes: test tube ①: 10 mg of medical-grade activated carbon powder; test tube ②: an empty tube.
[0022] When using it, you can follow the steps below: (1) Treatment of patient’s plasma: process the plasma according to the method of lupus anticoagulant detection in the laboratory, or according to the instructions of the lupus anticoagulant detection reagent (centrifuge at 1500gl for 10min, repeat twice). (2) Take out 500-1000 microliters of the processed plasma directly and place it in the test tube ①, and mix it upside down at room temperature for 5 minutes. (3) Centrifuge the test tube ① for 2 minutes, take the supernatant and put it in the test tube ②, then centrifuge the test tube ② for 2 minutes, the supernatant after centrifugation is the plasma without rivaroxaban, which can be directly used for the detection of lupus anticoagulant substances .
Embodiment 2
[0023] Embodiment 2 condition screening experiment
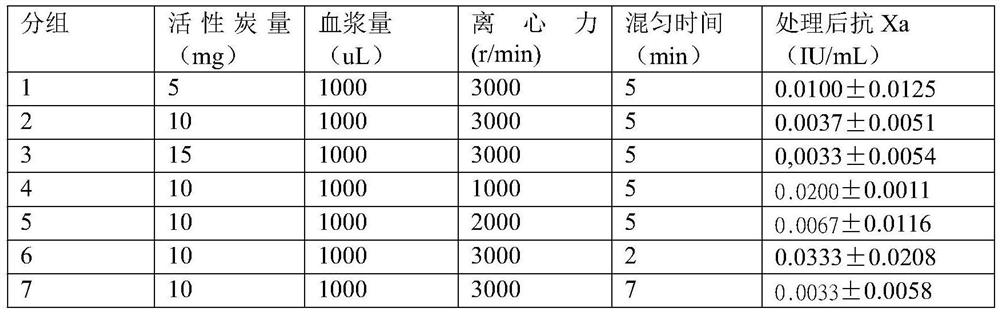
[0024] Screening of the ratio of activated carbon to plasma, mixing time and centrifugal force:
[0025] The ratio of activated carbon and plasma in the detection method in Example 1, the mixing time of activated carbon and plasma, and the centrifugation conditions in step 3 are replaced accordingly according to those in Table 1, and the other steps are reported unchanged to process plasma and process After the plasma was tested for lupus anticoagulant substances. Among them, the ratio of activated carbon to plasma (5:1000; 10:1000; 15:1000), mixing time (2min; 5min; 7min), centrifugal force (1000r / min; 2000r / min; 3000r / min). Each group was repeated 20 times, and the results are shown in Table 1. It was concluded that there was no difference in the removal efficiency of rivaroxaban between the two ratios of 10:1000 and 15:1000. The amount is less than 10:1000. For different centrifugal forces, the removal rate is obviousl...
Embodiment 3
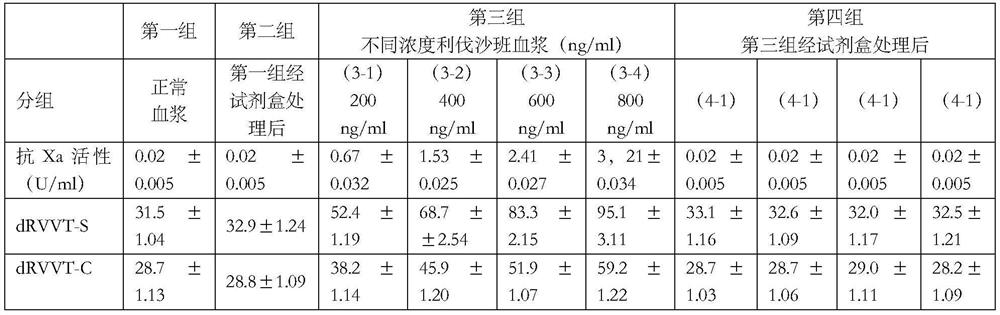
[0029] 1) Normal fresh frozen plasma obtained from the blood transfusion department (the plasma does not contain rivaroxaban or lupus anticoagulant substances), and about 10-15 parts each time are mixed into normal mixed plasma, and a total of 20 mixed plasmas are obtained, each 10ml. After two times of centrifugation at 1500g for 10 minutes, the supernatant was obtained to obtain platelet-poor plasma (PLT<10*109 / L).
[0030] 2) The platelet-poor mixed plasma obtained in step 1) was divided into four groups, the first group took 1ml without any treatment; the second group took 1ml and carried out kit treatment by the method described in Example 1; the third group was Prepare 2ml each of rivaroxaban plasma (200ng / ml, 400ng / ml, 600ng / ml, 800ng / ml) with four concentrations of rivaroxaban standard substance. After preparation, take 1ml of each concentration and use Example 1 As the fourth group after the kit treatment was carried out according to the method described in .
[003...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com