Lupus anticoagulant detection reagent as well as preparation method and use method thereof
A lupus anticoagulant and detection reagent technology, applied in the field of biological detection reagents, can solve the problems of increased false positive rate and false negative rate of test results, no LA, poor stability, etc., to reduce false positive rate and false negative rate, The effect of improving accuracy and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
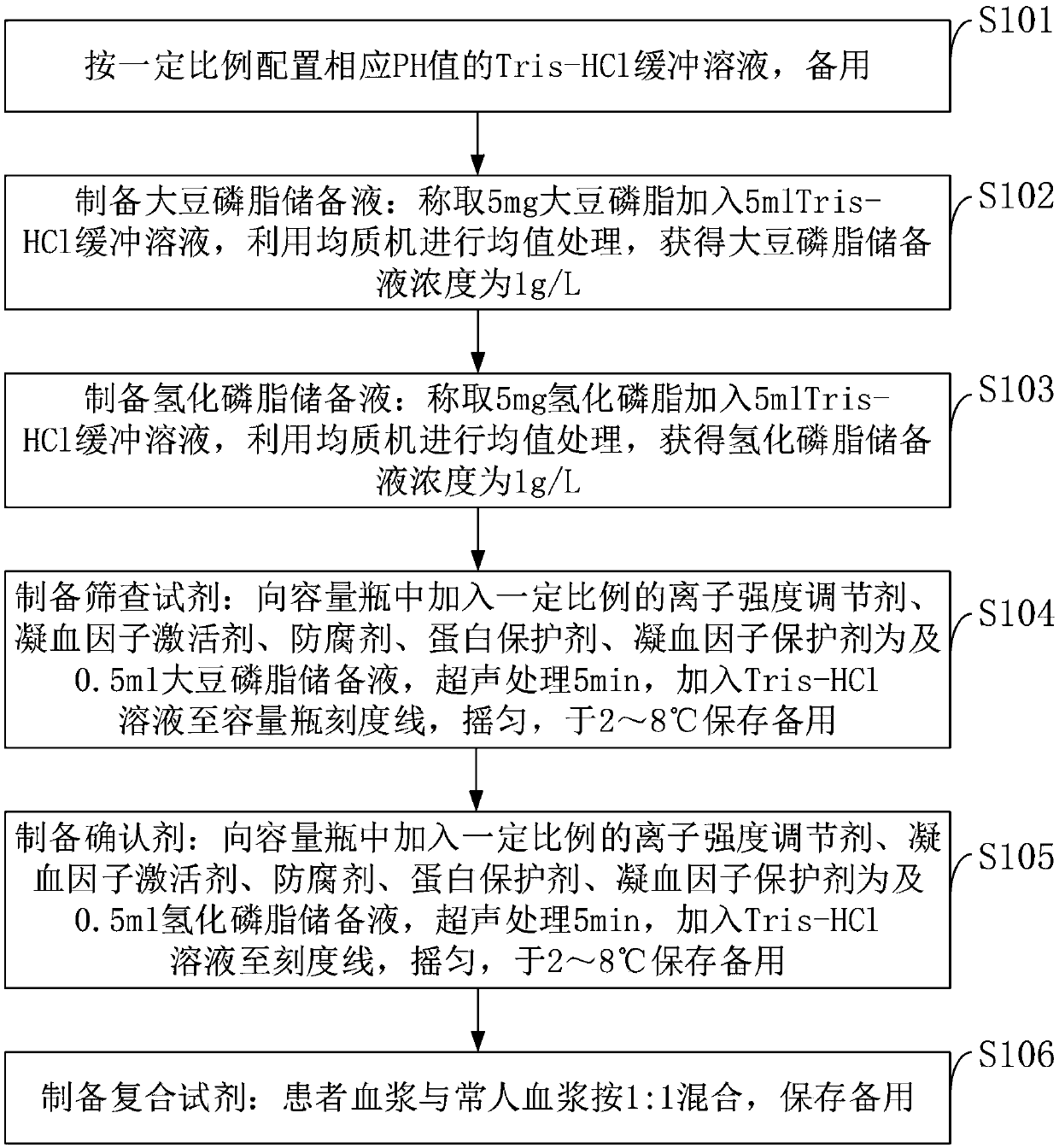
[0045] Such as figure 1 As shown, a preparation method for detecting lupus anticoagulant reagent provided by the embodiment of the present invention specifically includes the following steps:
[0046] S101: configure the Tris-HCl buffer solution of the corresponding pH value in a certain proportion, for subsequent use;
[0047] S102: Prepare soybean lecithin stock solution: Weigh 5 mg soybean lecithin and add 5 ml Tris-HCl buffer solution, use a homogenizer to perform mean value processing, and obtain a concentration of soybean lecithin stock solution of 1 g / L;
[0048] S103: Prepare hydrogenated phospholipid stock solution: Weigh 5 mg of hydrogenated phospholipid and add 5 ml of Tris-HCl buffer solution, use a homogenizer to perform mean value processing, and obtain a hydrogenated phospholipid stock solution with a concentration of 1 g / L;
[0049] S104: Preparation of screening reagents: add a certain proportion of ionic strength regulator, coagulation factor activator, pres...
Embodiment 1
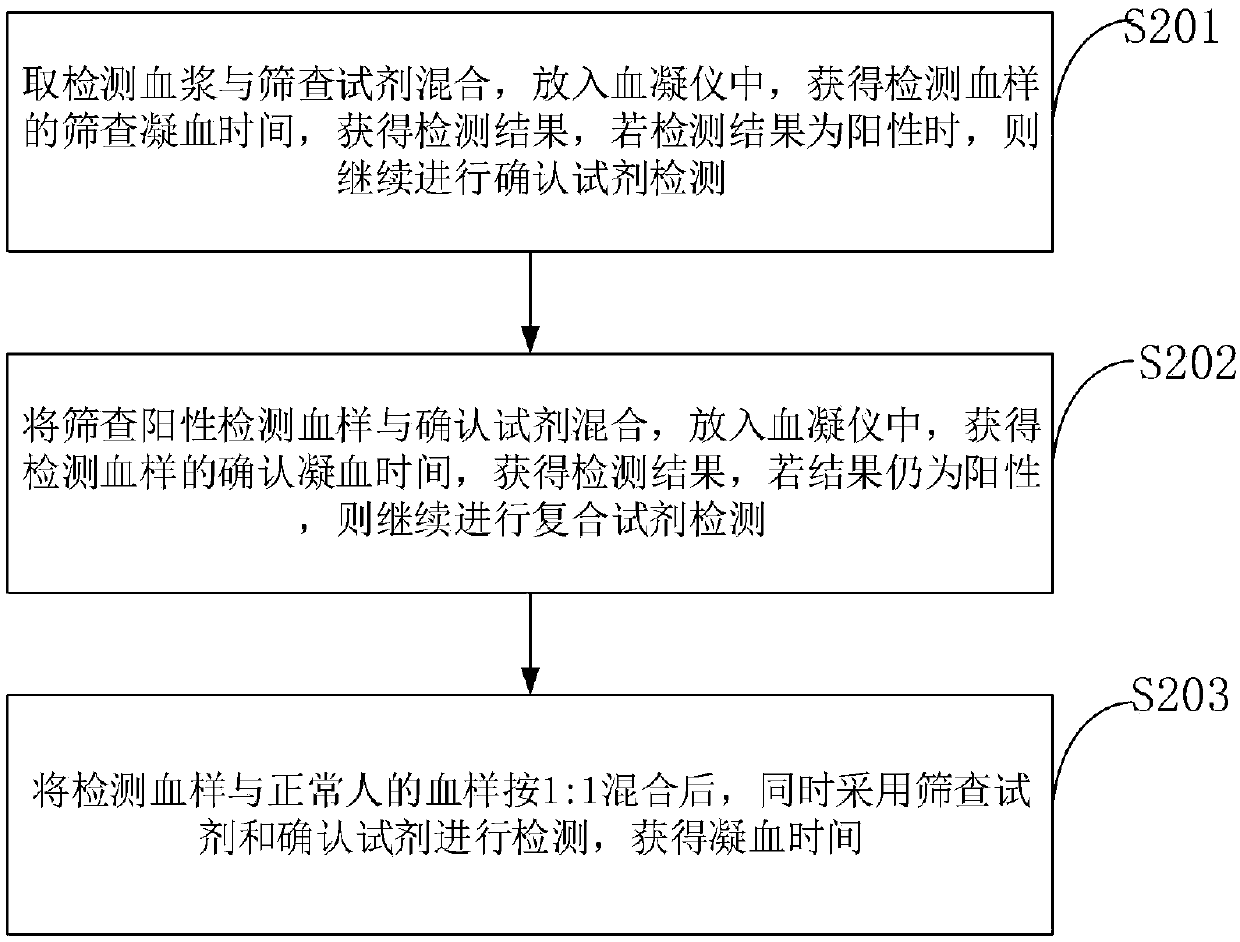
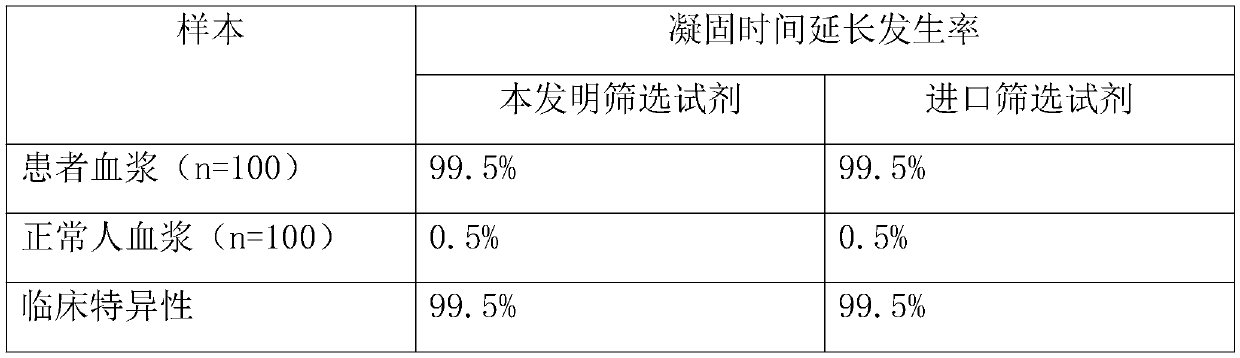
[0061] With the test kit prepared by the present invention and imported other brands of LA detection reagents, 200 cases of clinical plasma samples were respectively screened and measured for LA, and the incidence of prolongation of plasma coagulation time was counted to investigate the clinical specificity of the reagents. The results are shown in Table 1. .
[0062] Table 1
[0063]
[0064] As can be seen from Table 1, the clinical specificity of the kit of the present invention reaches the standard of imported reagents.
Embodiment 2
[0066] The kit prepared by the present invention was stored at 6°C, taken out when used, and the coagulation time of the same LA normal plasma and LA abnormal plasma was measured at different times, and the results are shown in Table 2.
[0067] Table 2
[0068]
[0069]
[0070] As shown in Table 2, the kit of the present invention has been continuously measured for 15 days, and the results have remained stable, indicating that the kit of the present invention has better stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com