External diagnostic reagent kit used for measuring activated partial thromboplastin time
A technology of thromboplastin time and thromboplastin, which is applied in the field of in vitro diagnostic kits, can solve the problems of increased cost of medical units, poor stability of reagents, and increased burden on patients, and achieves reduction of medical costs, good consistency, and wide application. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 Application of kit of the present invention
[0024] 1. Detection principle
[0025] Partial thromboplastin solution was added to the plasma to be tested, and at Ca 2+ Participate in the transformation of fibrinogen into insoluble fibrin, and measure the time required for coagulation, which is the activated partial thromboplastin time (APTT) of the plasma to be measured.
[0026] 2. Detection steps
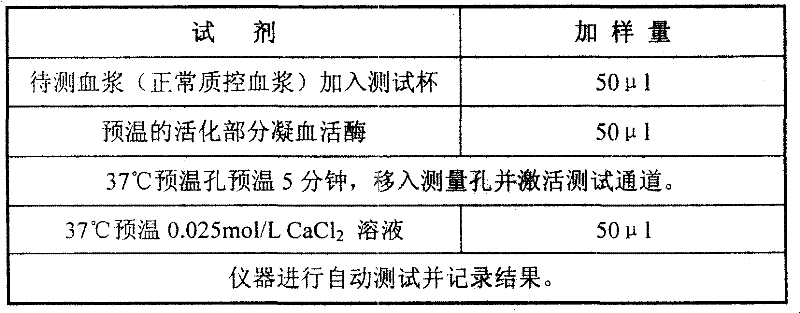
[0027] Part of the thromboplastin reagent was shaken with a certain volume of distilled water to dissolve into a suspension, and pre-warmed at 37°C. Taking the Pacific TS400C coagulation analyzer as an example, the operation steps are shown in Table 1:
[0028] Table 1
[0029]
[0030] When measuring with other hemagglutination instruments, operate according to the parameters provided by the corresponding instrument instructions.
Embodiment 2
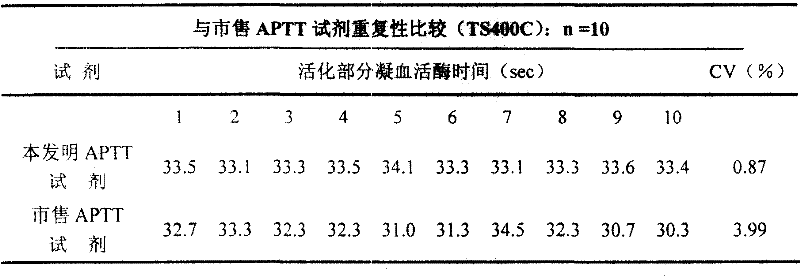
[0031] Embodiment 2 is compared with commercially available APTT reagent repeatability
[0032] Add 1 g of rabbit brain powder dehydrated with acetone to 20 ml of extract (chloroform), place the mixed solution on a constant temperature stirrer and stir for 3 hours, pour it into a sand core funnel and filter, and use a circulating water vacuum pump to remove the chloroform in the filtrate. All are drawn off to extract cephalin (partial thromboplastin). Dissolve cephalin with distilled water, mix and dissolve with the following raw materials: activator 0.01-0.3%, sodium chloride 0.5-10%, glycine 1-3%, carbolic acid 1.5-4%, NaN 3 1‰, stir evenly, freeze-dry after aliquoting.
[0033] When in use, the freeze-dried partial thromboplastin reagent was reconstituted with a certain volume of distilled water, and the activated partial thromboplastin time (APTT) of the same normal quality control plasma (APTT value: 25-45 seconds) was measured on the Pacific TS400C coagulation analyzer....
Embodiment 3
[0037] Embodiment 3 compares with commercially available APTT reagent stability
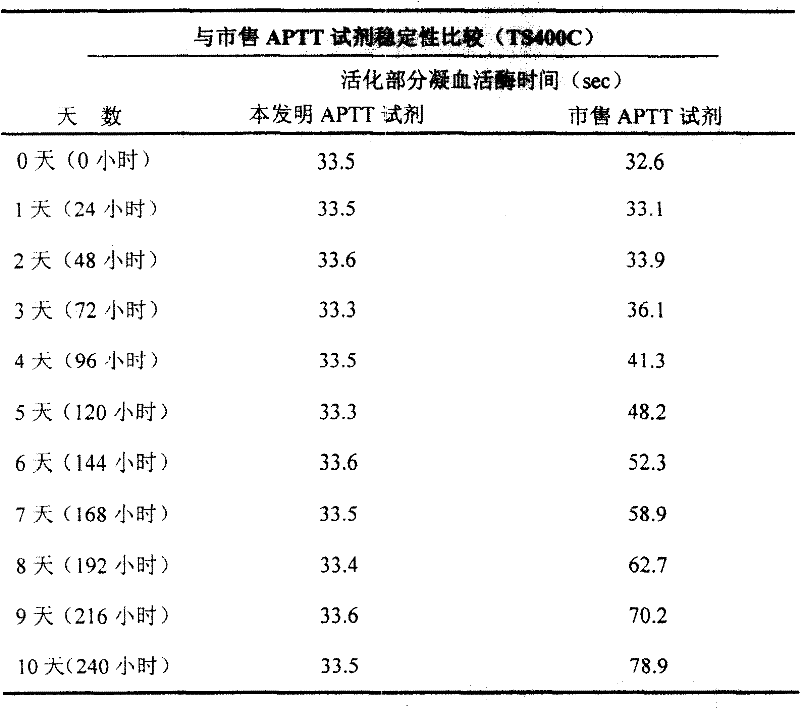
[0038] Part of the freeze-dried product of thromboplastin reagent was reconstituted with a certain volume of distilled water, and then the reconstituted reagent was stored at 37°C. The same normal quality control plasma (APTT value: 25-45 seconds) was measured on the Pacific TS400C coagulation analyzer, and the commercially available reagents were measured simultaneously, see Table 3.
[0039] Table 3 shows that the APTT reagent of the present invention is continuously measured for ten days and the results are still relatively stable, while commercially available reagents start to change on the third day. This result is very important for clinical laboratories because it can reduce waste, thereby reducing cost.
[0040] table 3
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com