Lupus anticoagulant confirmation kit (coagulation method)
A lupus anticoagulant and reagent technology, applied in the field of clinical diagnosis, can solve the problem of no "gold standard"
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Effects of Different Phospholipids on the Sensitivity of Confirmation Kits
[0038] The present invention has compared soybean lecithin and bovine cephalin, and the concentration is 30 μ g / mL, and formula is as follows:
[0039] 50mM Tris-HCl buffer at pH 7.30, 30μg / mL soybean lecithin / bovine cephalin, 0.2U / mL viper venom activator RVV-X, 25mM calcium chloride, 2μg / mL polybrene, 1% PEG6000;
[0040] At the same time, NPP (NPP, normal pooled plasma, normal pooled plasma) and LA positive samples were detected, and soybean phospholipids were found to have better sensitivity. The results are shown in Table 1.
[0041] Table 1
[0042]
[0043]
Embodiment 2
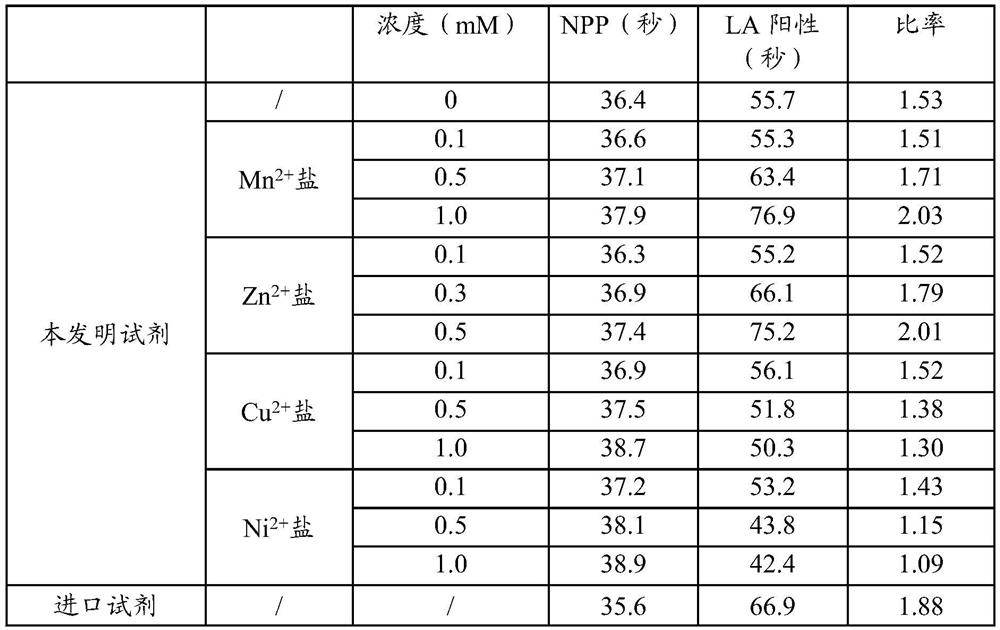
[0044] Embodiment 2: the impact of different metal ions on the sensitivity of the kit
[0045] Table 2 is respectively based on the formula of embodiment 1 (soybean lecithin) with different concentrations of Zn 2+ , Mn 2+ 、Cu 2+ 、Ni 2+ The results obtained by the confirmation reagent of the present invention for detecting NPP and LA positive plasma are compared with the simultaneous detection of imported kits, wherein each metal salt is its chloride, but not particularly limited.
[0046] Table 2
[0047]
[0048] It can be seen from Table 2 that adding Mn 2+ Salt or Zn 2+ After adding salt, the sensitivities of the reagents all increased, and with the increase of the concentration, the sensitivities were also higher. Join Cu 2+ Salt and Ni 2+ After adding salt, the sensitivity of the reagent decreased significantly, and the sensitivity decreased more obviously as the concentration increased.
Embodiment 3
[0049] Example 3: Comparison of clinical sample detection between the kit of the present invention and the imported kit
[0050] Based on the comparison results of the metal ions in Example 2, the confirmation kit of the present invention with the addition of 0.4mM zinc chloride and the imported kit were used to measure 200 clinical samples at the same time. The negative and positive results detected by the two kits are shown in Table 3. .
[0051] table 3
[0052]
[0053] The negative coincidence rate of this kit calculated according to the above table=90 / (90+16)×100%=84.9%, the positive coincidence rate=90 / (90+4)×100%=95.7%, the total coincidence rate= (90+90) / 200×100%=90%, and the rank sum test shows that the detection difference between the two kits has no statistical significance, indicating that the diagnostic sensitivity of the kit of the present invention has reached the imported reagent standard.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com