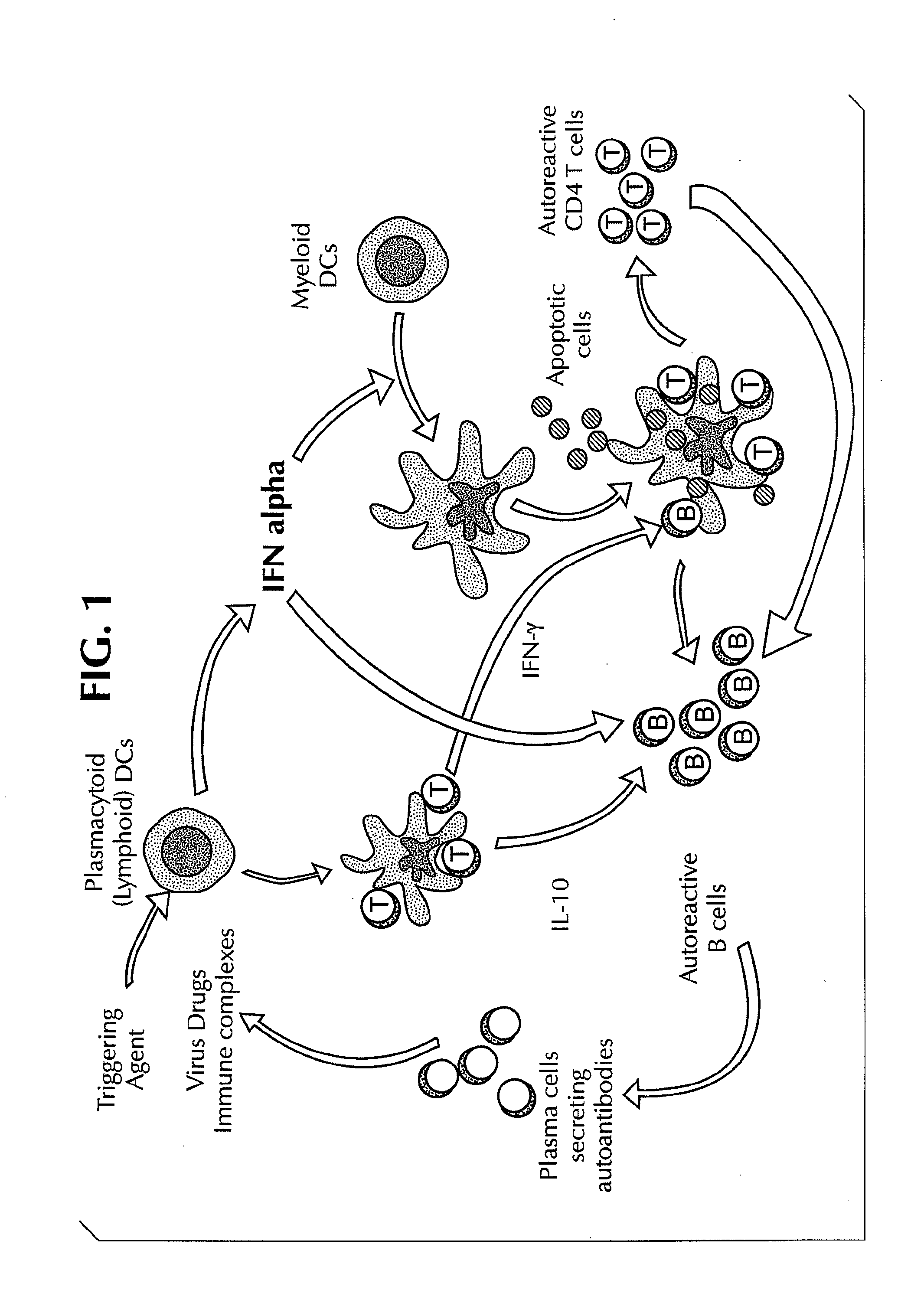
Methods for Treating Autoimmune Diseases in a Subject and In Vitro Diagnostic Assays
a technology for autoimmune diseases and in vitro diagnosis, applied in the direction of antibody medical ingredients, liposomal delivery, peptide/protein ingredients, etc., can solve the problems of devastating and crippling diseases of autoimmune diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
SLE Sera Induces Monocytes to Differentiate into Cells with Properties Like Dendritic Cells
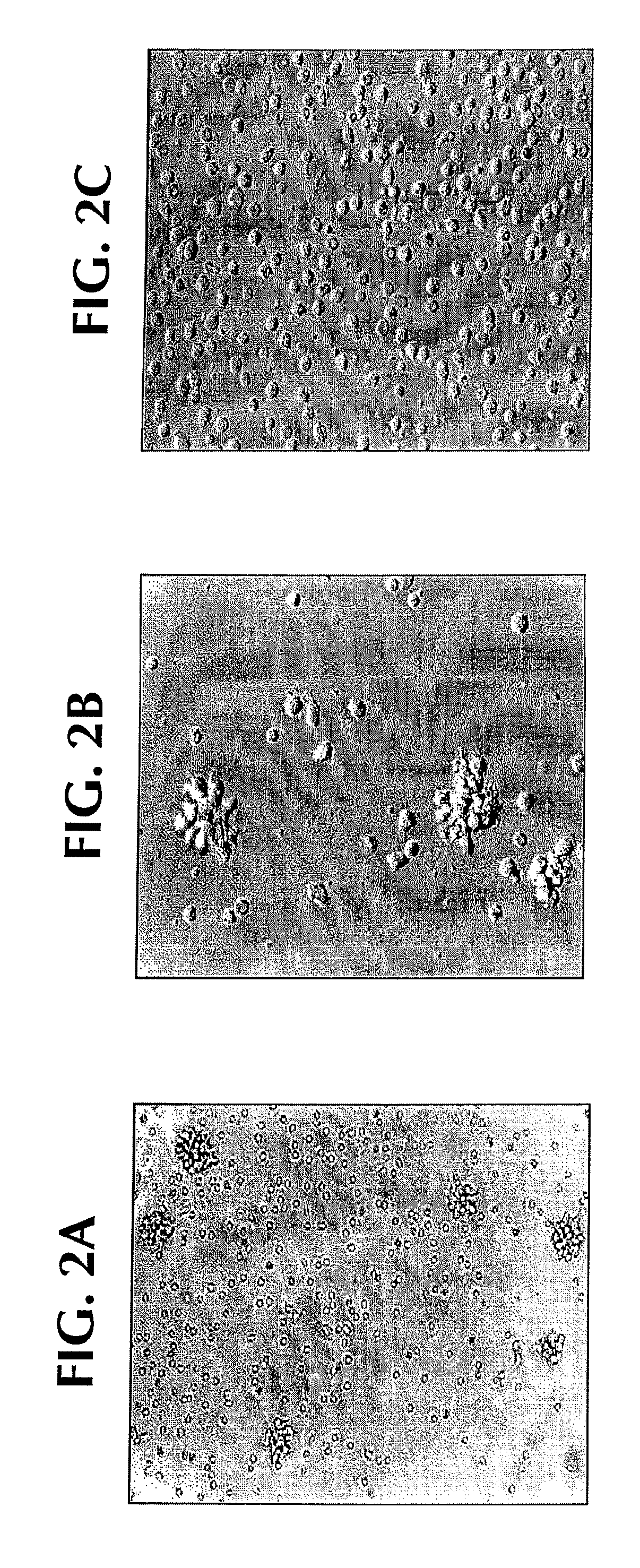
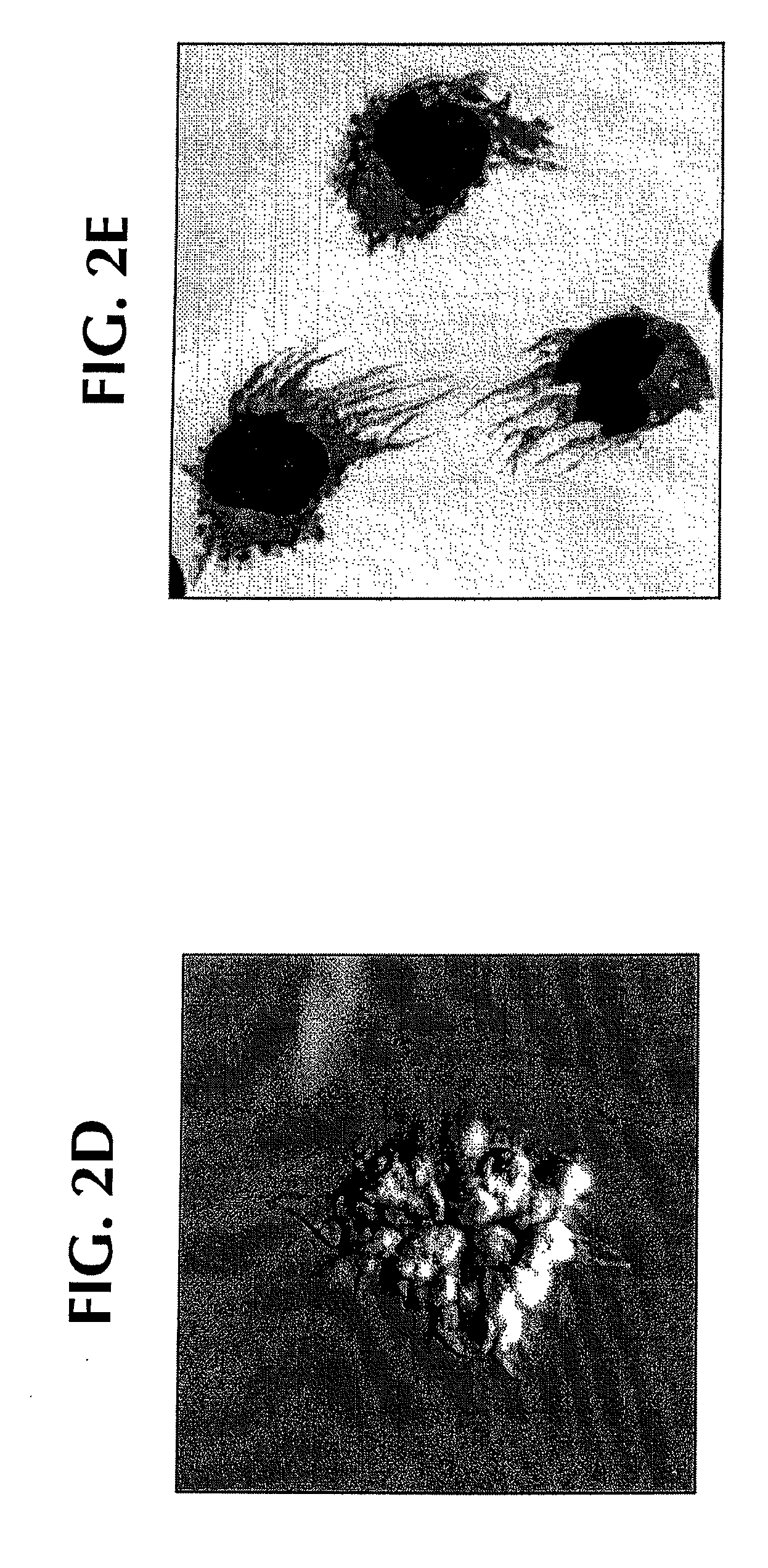
[0117]Normal monocytes were exposed to SLE sera in vitro: within 12-24 hours, clustering of monocytes was noted; and within 24-48 hours, clustered cells displayed fine cytoplasmic projections reminiscent of DC cultures (FIGS. 2A and 2B). Only SLE serum induced monocytes to cluster with 12-24 hours and acquire veiled cell morphology (FIGS. 2A-2B compared to AS in FIG. 2C).
[0118]SLE serum: After informed consent, blood was obtained from the patient who satisfied diagnostic criteria of American College of Rheumatology (ACR) for SLE. Whole blood was collected into tubes containing EDTA or heparin, and was separated immediately by centrifugation at 100×g at 4° C. The plasma was harvested, treated with thrombin (Jones Pharma Incorporated, MO) and stored at −80° C. until used. Disease activity was assessed by using the SLUE Disease Activity Index (SLEDAI) score (Lahita, R. G. 1999. Systemic Lupus Ery...
example 2
SLE-DCs Present Antigens from Captured Apoptotic Cells
[0127]The non-normal and inappropriate processing of apoptotic cells by the immune system is considered as one of the pathogenic events in SLE. Thus, it was next determined whether SLE-DCs can present antigens from captured apoptotic cells. To this end, SLE-DCs were shown to be able to capture apoptotic cell fragments in culture (FIGS. 7A-7B).
[0128]SLE-DCs could also capture DNA containing apoptotic bodies derived from melanoma cells. FIGS. 8A and 8B show the capture of allogeneic apoptotic cells and presentation of their antigens to autologous CD4+ T cells. SLE-DCs captured DNA containing apoptotic bodies (FIG. 8A) and presented their antigens to autologous CD4+ T cells as indicated by the induction of CD4+ T cell proliferation (FIG. 8B). HLA-DR+ monocytes induced by AS serum SLE serum and GM-CSF / IL-4 captured 7AAD labeled DNA-bodies (melanoma cell line killed by gamma-irradiation (150 Gy)). To allow capture of apoptotic bodies,...
example 3
Only IFN-α Containing Active SLE Sera Induce Monocytes to become SLE-DCs
[0130]Experiments were performed to determine whether all SLE sera were able to direct monocyte differentiation into DCs. Monocytes were cultured with 19 different SLE sera to be tested for their ability to stimulate a mixed leukocyte response or mixed lymphocyte reaction (MLR). As shown in Table 1, monocytes cultured with autologous serum were only able to induce very low T cell proliferation (negative control), and monocytes cultured with GM-CSF / IL-4 to produce DCs elicited a 100% MLR (positive control), the mean proliferation was 7.5% (±6.4% Y, n=5). When monocytes cultured with SLE sera were evaluated in the same way, considering 20% proliferation as a cut-off point (mean+2SD of autologous serum cultured monocytes) 11 of the 19 sera induced monocyte-s to become allostimulatory DCs with mean proliferation of 41%±15%. As sera from patients with dermatomyositis were unable to reproduce this skewing and since th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com