Stable biochemical compound calibrator and preparation method thereof
A calibrator and biochemical technology, applied in the field of biological in vitro diagnosis, can solve the problems of inconvenient clinical inspection work and few analysis items, and achieve the effect of long storage period, lower production cost and avoid infection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A stable biochemical compound calibrator whose matrix is serum and whose raw materials contain the following components:
[0053] 1) Protective substances:
[0054] Sucrose content 50g / L, mannitol content 50g / L, ascorbic acid content 1.0mmol / L
[0055] Octylphenoxyethylene ether content 0.50% (w / v) bovine serum albumin content 10.0g / L
[0056] NaN 3 Content 0.10% (w / v)
[0057] 2) Analytical components:
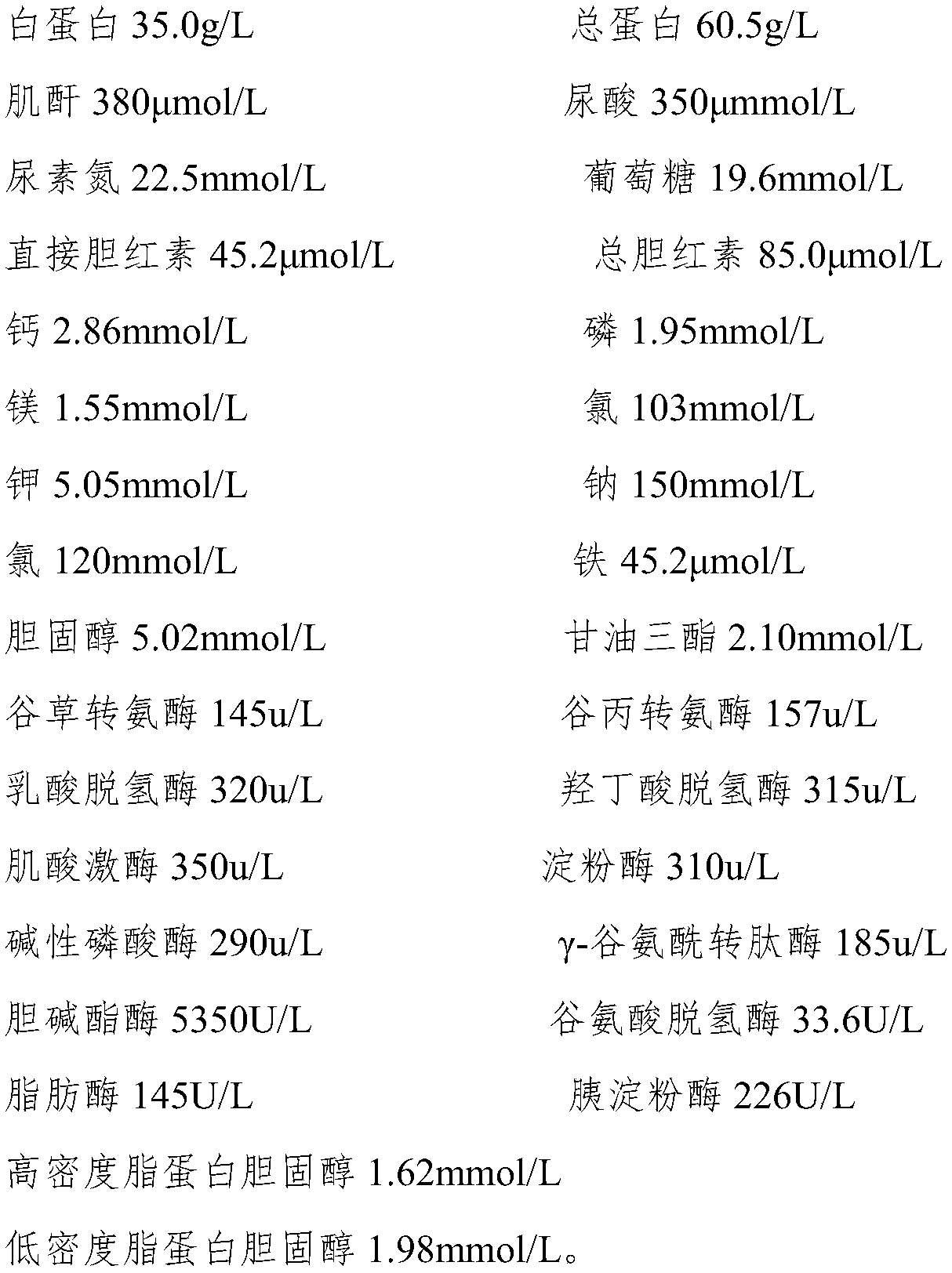
[0058]
[0059]
[0060] The preparation method of this stable biochemical composite calibrator comprises the steps:
[0061] 1) Collect waste blood from clinical examination, requiring hepatitis B virus surface antigen HBsAg, hepatitis C virus HCV antibody, human immunodeficiency virus HIV-1 and HIV-2 antibody, and syphilis to be negative;
[0062] 2) The serum was separated by centrifugation and filtered through a 0.2 μm filter membrane as a spare serum matrix;
[0063] 3) Analyze and measure the content of each calibration component in the serum accordin...
Embodiment 2
[0076] A stable biochemical compound calibrator whose matrix is serum and whose raw materials contain the following components:
[0077] 1) Protective substances:
[0078] Trehalose content 50g / L, sorbitol content 50g / L, butylated hydroxyanisole content 1.0mmol / L
[0079] Triton-100 content 0.50% (w / v) bovine serum albumin content 10.0g / L
[0080] NaN 3 Content 0.10% (w / v)
[0081] 2) Analytical components:
[0082]
[0083] The preparation method of this stable biochemical composite calibrator comprises the steps:
[0084] 1) Collect waste blood from clinical examination, requiring hepatitis B virus surface antigen HBsAg, hepatitis C virus HCV antibody, human immunodeficiency virus HIV-1 and HIV-2 antibody, and syphilis to be negative;
[0085] 2) The serum was separated by centrifugation and filtered through a 0.2 μm filter membrane as a spare serum matrix;
[0086] 3) Analyze and measure the content of each calibration component in the serum according to conventi...
experiment example
[0098] In order to study the influence of the protective agent on the stability of the calibrator and the resolubility of the product, after adding each analysis component (same as Example 1), design the formulas 1-8 in the following table 3, and the ninth group is used as the blank control serum. No protective agent is added, the product is prepared after freeze-drying and packaging, and stored in a 2-8°C refrigerator and a 37°C incubator respectively. The corresponding kits and calibrators of Zhongsheng Beikong Biotechnology Co., Ltd. were used to determine the content of each component in the product, and the influence of different protective agents on the stability and resolubility of the product was observed. The experimental results are shown in Table 3, Table 4 and Table 5.
[0099] The experimental results show that: in the accelerated test at 37°C for 14 days, the recovery rate of the calibrator added with the protective agent is greater than 75%, and the recovery rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com