hepatitis B surface antigen resistant antibody and application thereof
一种抗体、抗原的技术,应用在抗体、应用、抗病毒剂等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[1732] The invention will now be described with reference to the following examples, which are intended to illustrate the invention, but not to limit it.
[1733] Unless otherwise specified, the molecular biology experiment methods and immunoassay methods used in the present invention are basically with reference to J.Sambrook et al., Molecular Cloning: A Laboratory Manual, 2nd Edition, Cold Spring Harbor Laboratory Press, 1989, and F.M.Ausubel et al., Molecular Biology Experimental Guide, 3rd Edition, John Wiley & Sons, Inc., 1995. The method described in; restriction endonucleases were used in accordance with the conditions recommended by the product manufacturer. Those skilled in the art understand that the examples describe the present invention by way of example and are not intended to limit the scope of the claimed invention.
Embodiment 1
[1734] Example 1: Mouse monoclonal antibody 6D11 specifically binding to HBsAg and its humanization
[1735] 1.1: Characterization of mouse monoclonal antibody 6D11
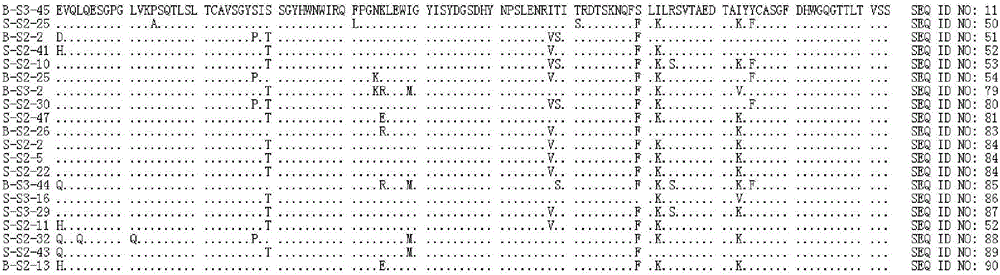
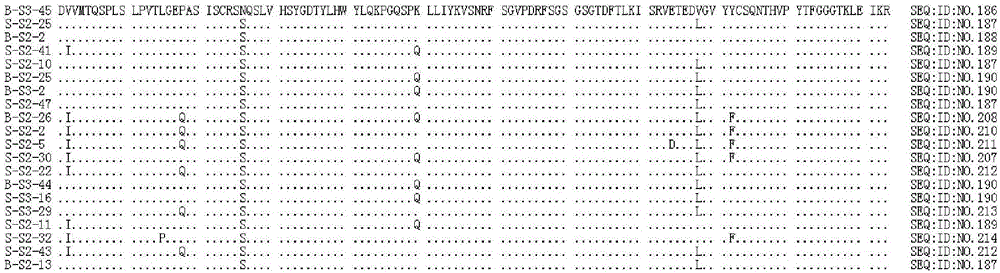
[1736] The mouse monoclonal antibody 6D11 (hereinafter also referred to as 6D11-mAb or mAb for short) that specifically binds to HBsAg has been prepared according to conventional immunological methods. The amino acid sequence of the heavy chain variable region of the mouse monoclonal antibody 6D11 is shown in SEQ ID NO:1, and the amino acid sequence of the light chain variable region is shown in SEQ ID NO:2.
[1737] SEQ ID NO: 1
[1738] DVQLQESGPGLVKPSQSLSLTCSVTGYPITSGYHWNWIRQFPGNKLVWMGYISYDGSDHYNPSLENRISITRDISKNQFFLILRSVTTEDTGKYFCASGFDHWGQGTTLTVSS
[1739] SEQ ID NO: 2
[1740]DVVMTQTPLSLPVSLGDQASISCRSSQSLVHSYGDTYLHWYLQKPGQSPKLLIYKVSNRFSGVPDRFSGSGSGTDFTLKISRVETEDLGVYFCSQNTHVPYTFGGGTKLEIKR
[1741] Further, the method described by Kabat et al. (Kabat et al., Sequences of Proteins of Immunological Interest...
Embodiment 2
[1760] Example 2: Single-point substitution of amino acids in the CDR region of humanized antibody B-S3-45
[1761] The amino acids at each site of the six CDR regions of the humanized antibody B-S3-45 were substituted with 20 naturally occurring amino acids at a single point. Using the cloning methods in Examples 1.3 and 1.4, the recombinant vector expressing the phage antibody was obtained, wherein the phage antibody had a single point mutation in the CDR region compared with B-S3-45. Amino acid substitutions at the mutation site were introduced using degenerate primers.
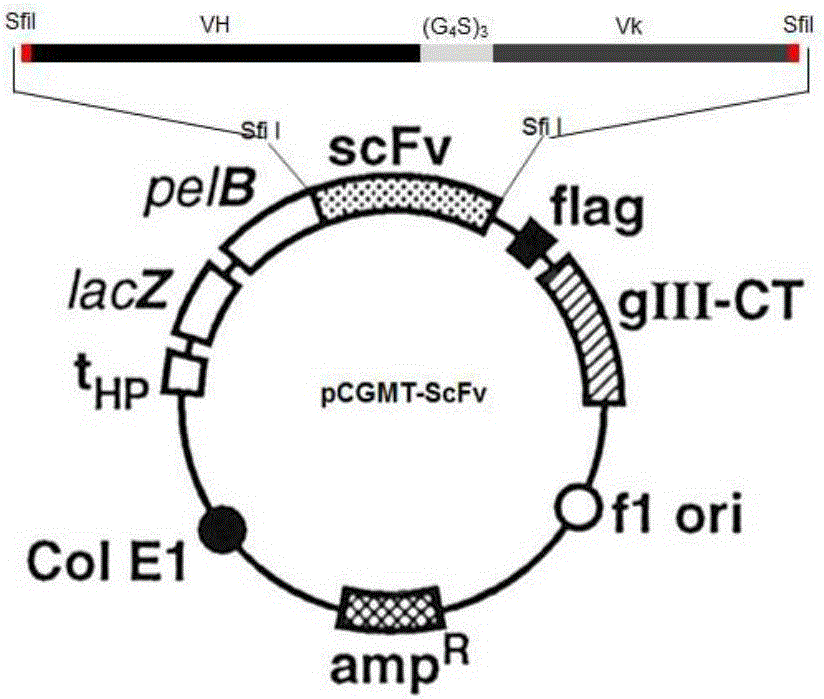
[1762] Taking the first amino acid of the heavy chain variable region CDR3 (HCDR3) as an example, oligonucleotide primers H3R1 and B45-H3F were designed (the sequences of which are shown in Table 5). For the annealing position on the coding gene, see Figure 4 . The specific protocol of PCR is as follows: using the coding gene of B-S3-45 as a template, primer pairs B45-VhF / H3R1 and B45-H3F / B45-VkR were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com