Antibodies for treatment of hepatitis B infection and related diseases
An antibody and antigen technology, applied in the direction of antibodies, introduction of foreign genetic material using vectors, antiviral agents, etc., can solve the problems of unstable nature, high price, and few sources of high-titer plasma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0468] The invention will now be described with reference to the following examples, which are intended to illustrate the invention, but not to limit it.
[0469] Unless otherwise specified, the molecular biology experiment methods and immunoassay methods used in the present invention are basically with reference to J.Sambrook et al., Molecular Cloning: A Laboratory Manual, 2nd Edition, Cold Spring Harbor Laboratory Press, 1989, and F.M.Ausubel et al., Molecular Biology Experimental Guide, 3rd Edition, John Wiley & Sons, Inc., 1995. The method described in; restriction endonucleases were used in accordance with the conditions recommended by the product manufacturer. Those skilled in the art understand that the examples describe the present invention by way of example and are not intended to limit the scope of the claimed invention.
Embodiment 1
[0470] Example 1: Preparation of human-cynomolgus monkey chimeric monoclonal antibody specifically binding to HBsAg
[0471] 1.1 Immunization of cynomolgus monkeys
[0472] Using the recombinant HBV vaccine previously developed by the laboratory (the recombinant vaccine is described in detail in Chinese patent application 201710085194.3) to immunize cynomolgus monkeys older than 6 months and weighing 4±1kg intramuscularly, the injection dose is 20μg / monkey / time. The immunization time points were at 0, 2, 6, 10, 14, 18, 22, and 26 weeks respectively. After the serum titer of cynomolgus monkey reached the plateau at the 4th dose of immunization (10 weeks), we produced a large number of memory B cells at 13, 17, 25, and 29 weeks (that is, the third week after each immunization), The peripheral blood of cynomolgus monkeys was collected 10ml / monkey / time.
[0473] 1.2 Isolation of peripheral blood mononuclear cells from cynomolgus monkeys
[0474] The isolation of peripheral ...
Embodiment 2
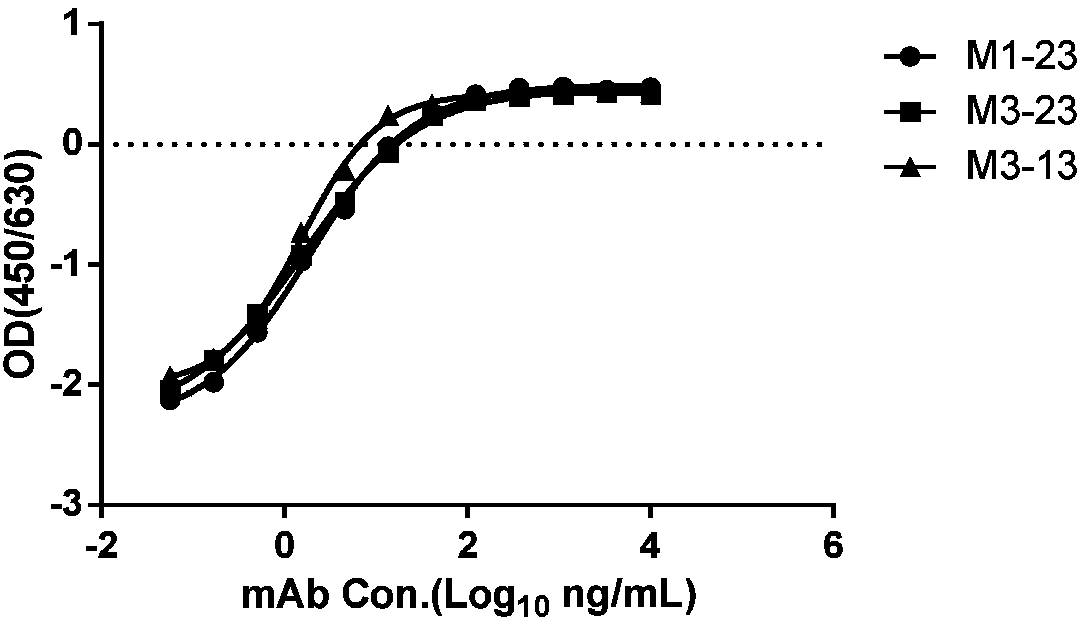
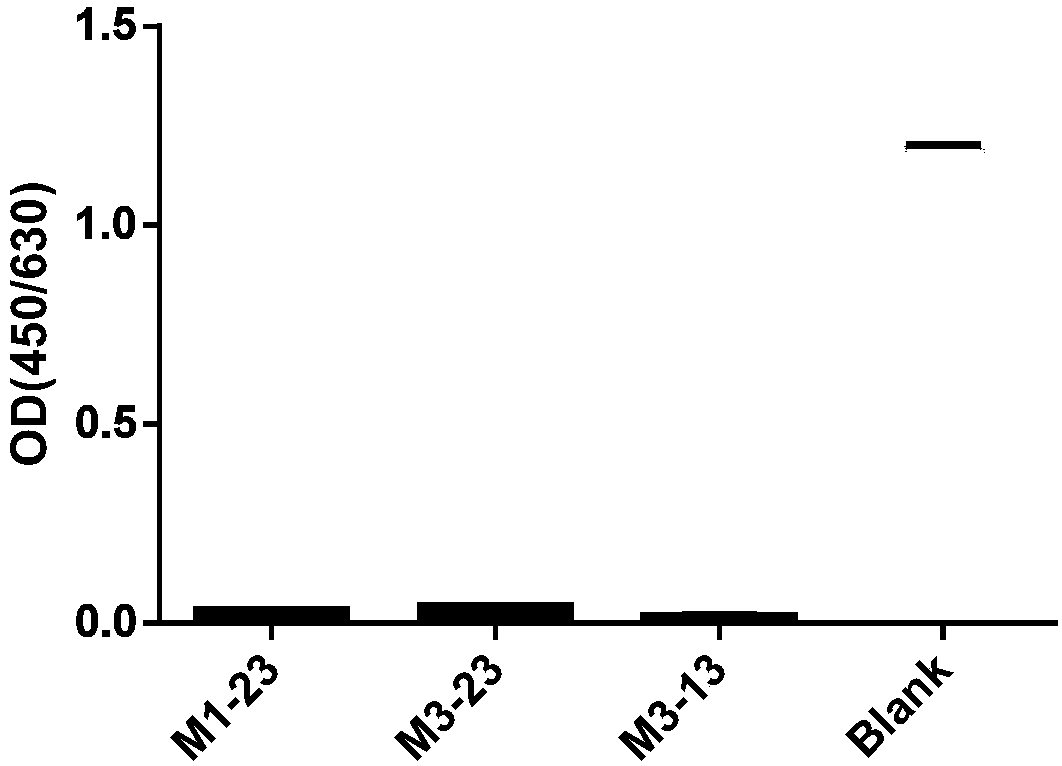
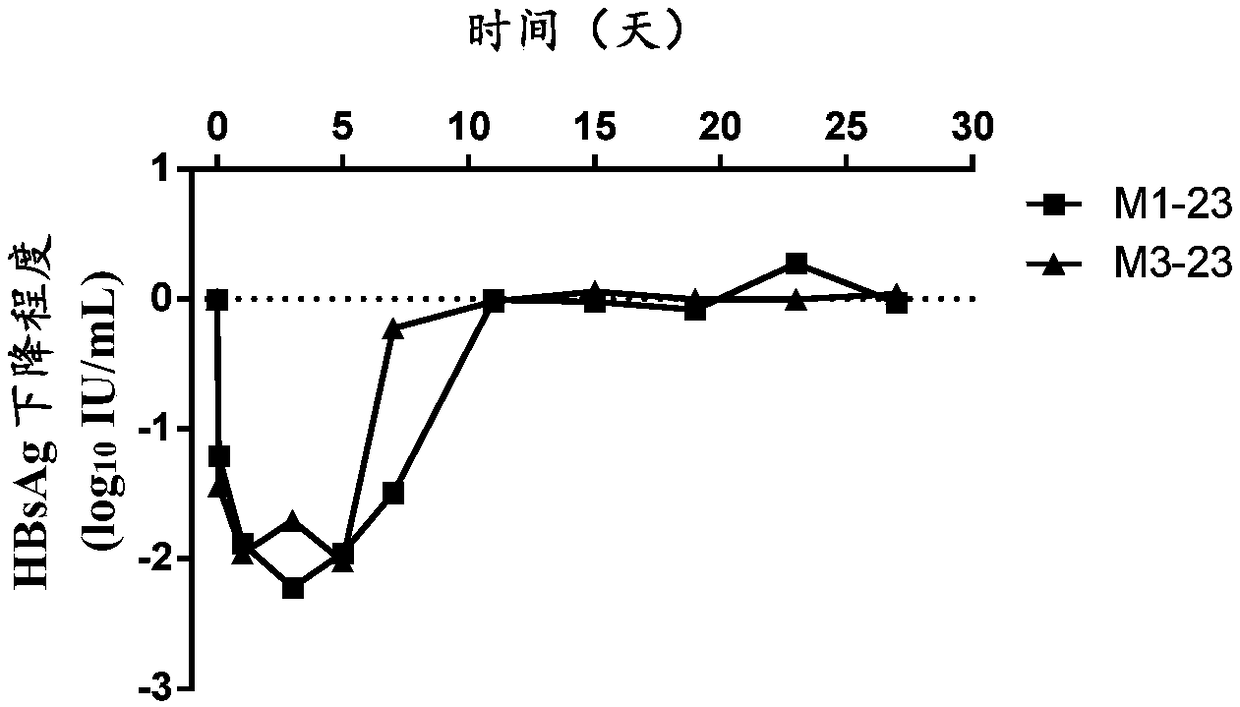
[0534] Example 2: Analysis of properties of cynomolgus monkey-human chimeric monoclonal antibody specifically binding to HBsAg
[0535] According to the method of Example 1, three strains of cynomolgus monkey-human chimeric monoclonal antibodies specifically binding to HBsAg were prepared and named M1-23, M3-23, and M3-13 respectively. The VH and VL amino acid sequences of the three antibodies are shown in the table below (SEQ ID NO: 1-5). In addition, the CDR sequences of the three antibodies were determined by using IMGT, and the amino acid sequences of the CDRs of the heavy chain variable region and the light chain variable region are shown in Table 5 (SEQ ID NO: 6-20).
[0536] Table 4: M1-23 / M3-23 / M3-13 light and heavy chain variable region amino acid sequences
[0537]
[0538]
[0539] Table 5: Sequences of M1-23 / M3-23 / M3-13 light and heavy chain CDRs
[0540]
[0541] The inventors conducted a series of property analyzes on the three purified monoclonal antibo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
| Solubility | aaaaa | aaaaa |
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com