Method for producing recombinant hepatitis B surface antigen
A technology of hepatitis B surface antigen and production method, which is applied in the production field of recombinant hepatitis B surface antigen, can solve the problems of VLP structure change, low yield, unstable purification process, etc., and achieve stable quality, short process cycle and high recovery rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
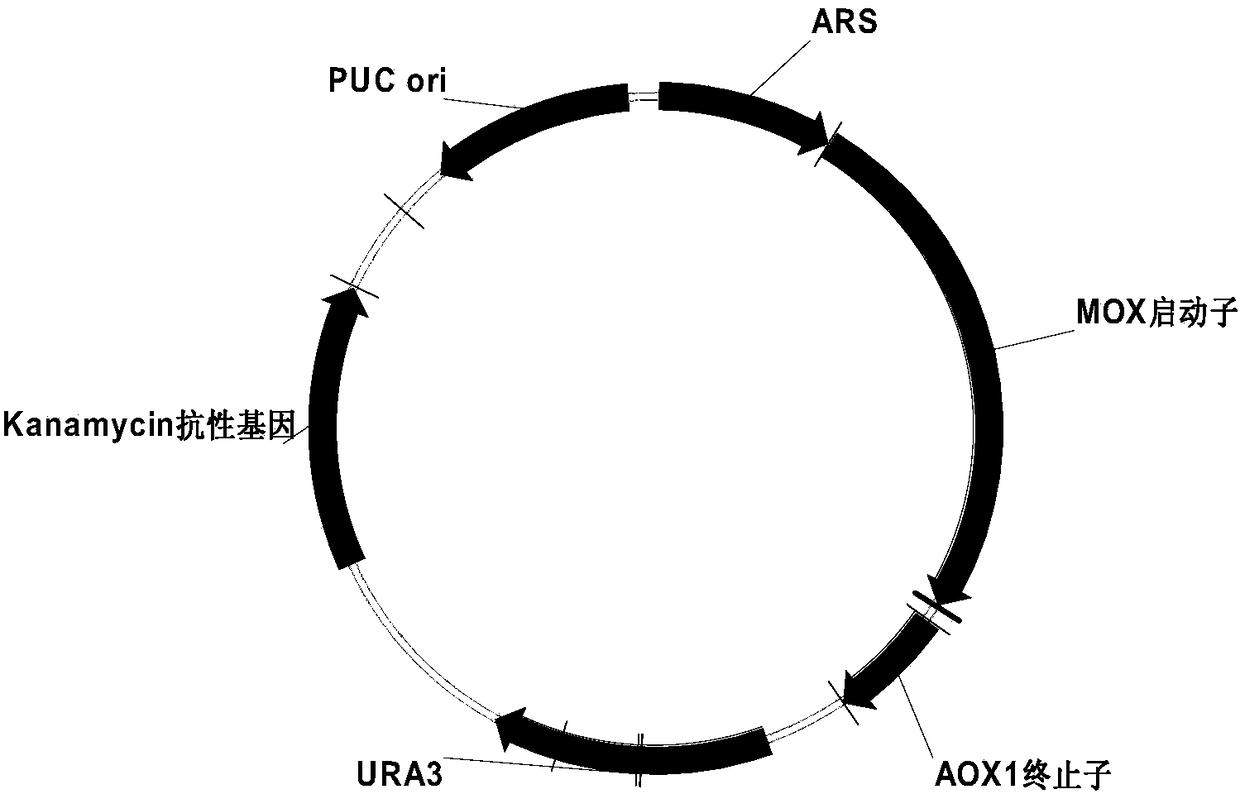
[0044] The construction of embodiment 1 expression vector pMAUR (S.C) KARS1
[0045] The primer sequences involved in the vector construction process are as follows:
[0046]
[0047]
[0048] 1) PCR amplification to obtain the MOX promoter sequence of methanol oxidase gene
[0049] Use the Yeast Genome Extraction Kit to extract Hansenula (ATCC 14754) genomic DNA, use the genomic DNA as a template, and use primer 1 and primer 2 to perform PCR reaction. The specific reaction conditions are: 50 μl reaction system, containing 1 μl KOD-Plus polymer Enzyme, 5μl 10×Buffer, 5μl dNTP, 2μl 25mM MgSO 4 , 1.5 μl primer 1, 1.5 μl primer 2, 1 μl template, 33 μl ddH 2 O. The reaction was carried out with a Bio-Rad PCR instrument. The reaction conditions were: 94°C for 2 minutes, cycle once; 94°C for 15 seconds, 55°C for 1 minute, 68°C for 1.5 minutes, a total of 35 cycles; 68°C for 1 minute, cycle 1 Second-rate. The PCR product was recovered and purified. The target fragment wa...
Embodiment 2
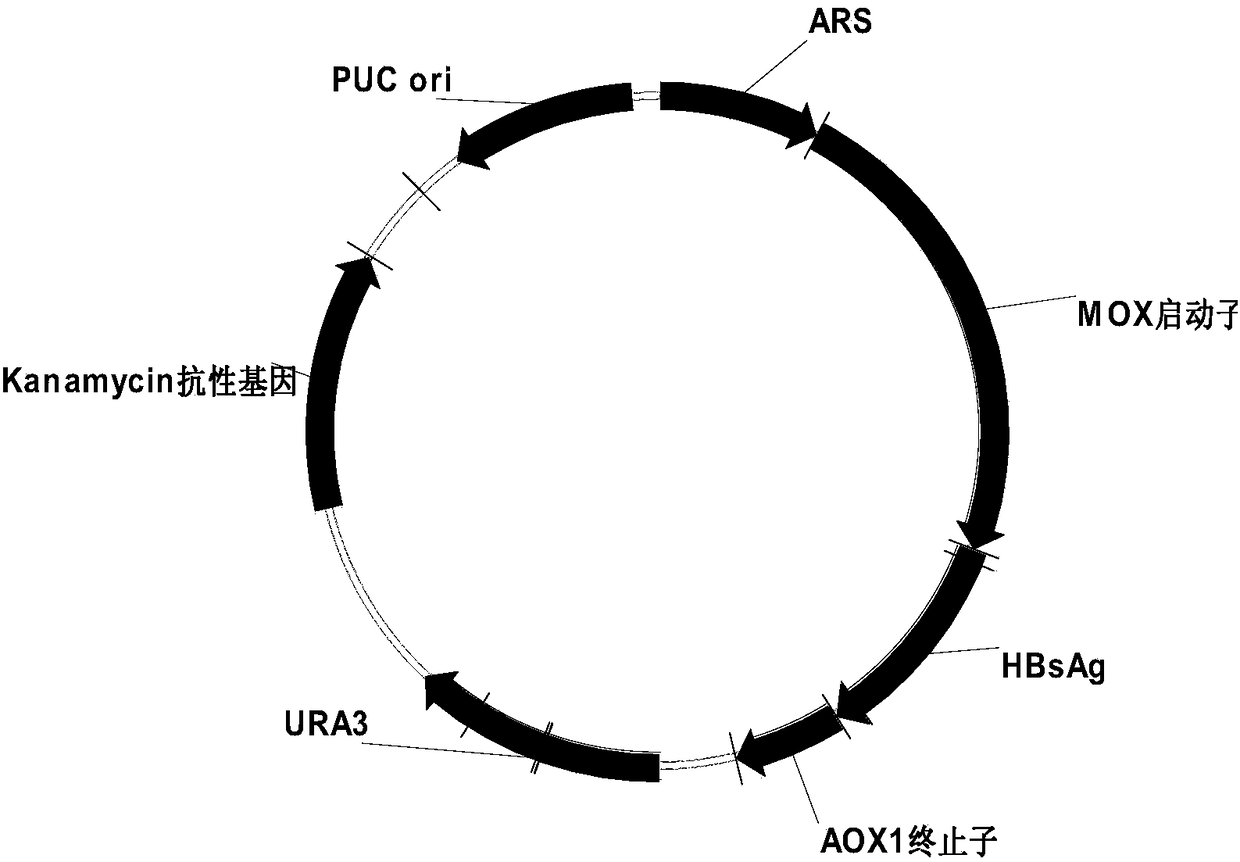
[0073] Example 2 Screening of Recombinant Hepatitis B Surface Antigen Hansenula High Expression Strain
[0074] 1) Transformation and screening of recombinant expression hepatitis B surface vector
[0075] Competent cell preparation: Pick a single clone of the URA3 auxotrophic Hansenula strain and culture it overnight at 37°C in 10mL of LYPD medium. Take 2 mL of the culture, inoculate it into a shaker flask containing 100 mL of YPD medium, and culture it at 37°C until OD600=1.3-1.5. Collect the cells by centrifuging at 3000g for 10 minutes at 4°C, and suspend the cells with 0.2 times the volume of pre-cooled 50mM phosphate buffer. Incubate at 37°C for 15 minutes. Centrifuge as above, suspend and wash the cells with 1 volume of pre-cooled STM buffer. Centrifuge as above, suspend and wash the cells with 0.5 volume of pre-cooled STM buffer. Centrifuge as above, and suspend the cells with 0.005 times the volume of pre-cooled STM buffer to a final volume of about 0.4 ml.
[0...
Embodiment 3
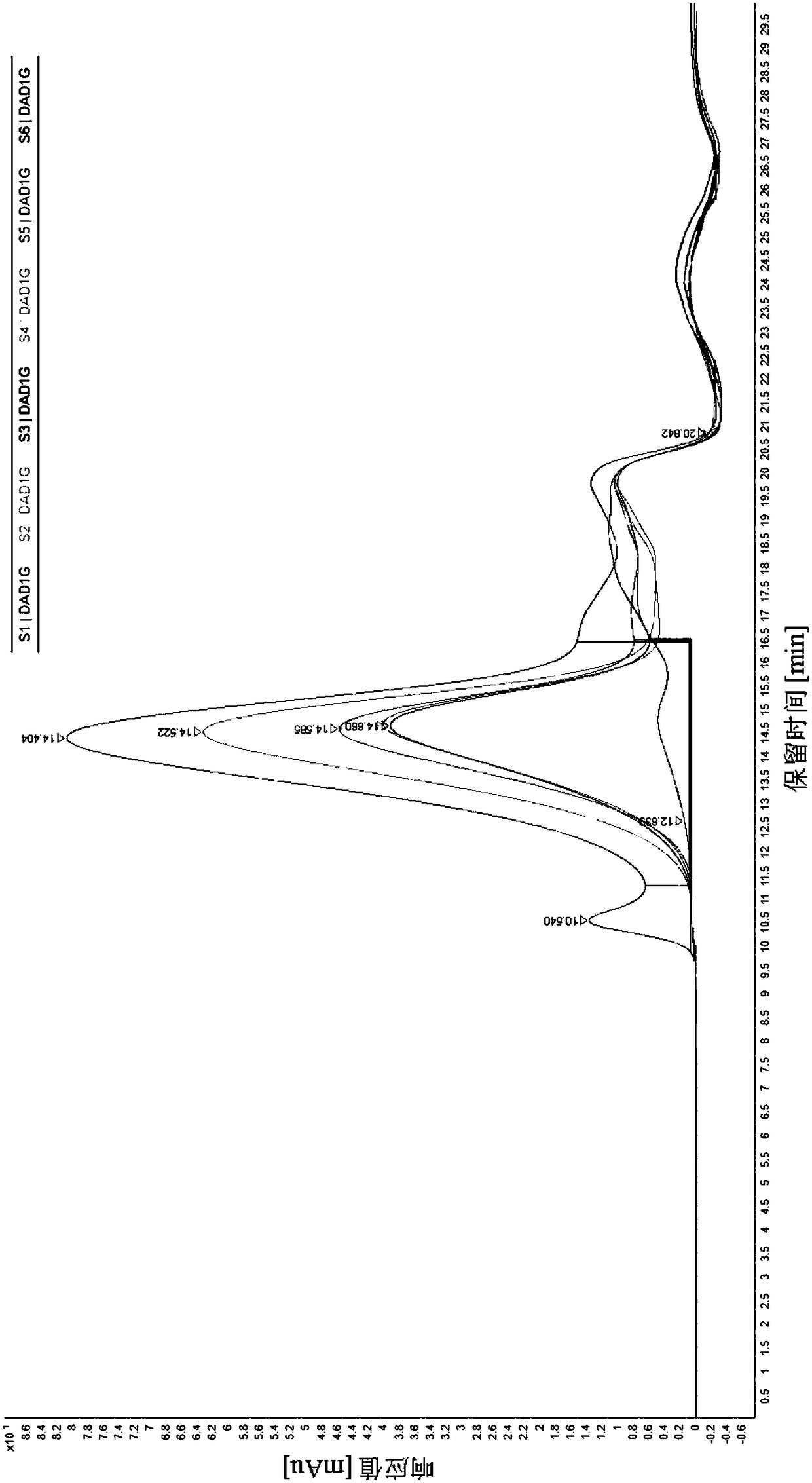
[0079] Example 3 Hansenula expresses recombinant hepatitis B surface antigen 50L bioreactor fermentation
[0080] 1) Preparation of first-level seed liquid: Take one of the above-mentioned recombinant Hansenula glycerol bacteria liquid (1.5mL / tube) and inoculate it into 200mL YPG medium, which contains: 20.00g / L glycerol, 10.00g / L yeast extract Material, 20.00g / L soytone. Adjust the rotation speed to 220rpm and culture at 37°C for 12 hours; when the OD600 reaches 10-15 hours, use it as the primary seed solution;
[0081] 2) Secondary seed solution preparation: the above-mentioned primary seed solution was inoculated in 2.0L BSM inorganic salt medium, and the BSM inorganic salt contained: 0.90g / L CaSO 4 .2H 2 O, 11.67g / L MgSO 4 .7H 2 O, 14.67g / L K 2 SO 4 , 9.00g / L (NH 4 ) 2 SO 4 , 50g / L glycerin and 25.05g / L sodium hexametaphosphate. Add 2mL of PTM1 trace elements per liter of BSM, PTM1 formula contains: 6.00g / L CuSO 4 .5H 2 O, 3.00g / L MnSO 4 .H 2 O, 0.02g / L H ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com