Use of lignan compounds for treating or preventing inflammatory disease
A technology of inflammatory diseases and lignans, applied in the field of pharmaceutical compositions for the treatment or prevention of inflammatory diseases, and the treatment or prevention of inflammatory diseases, and can solve problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
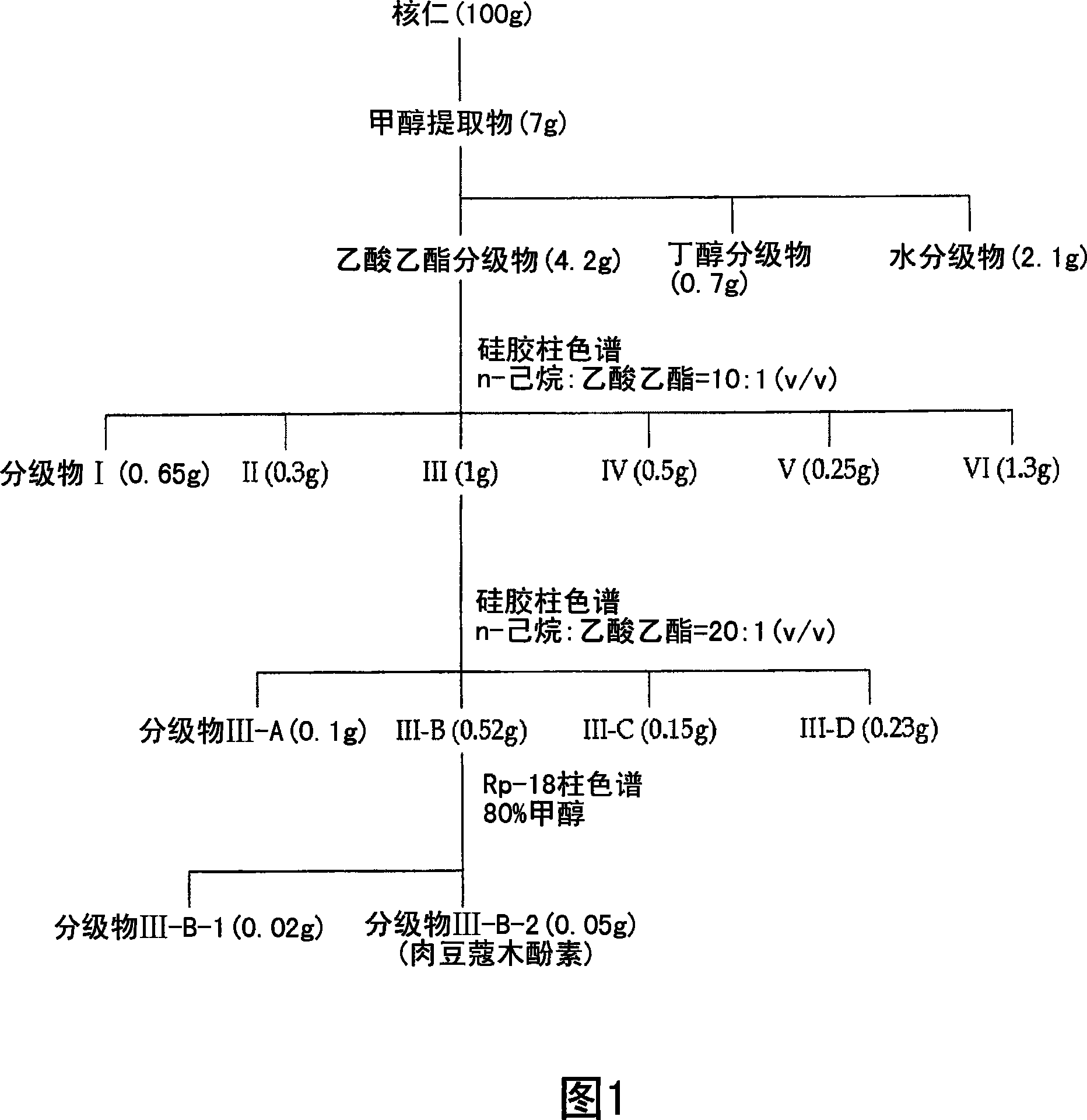
[0072] Isolation and purification of lignans from nutmeg
[0073] Separation and purification of lignans
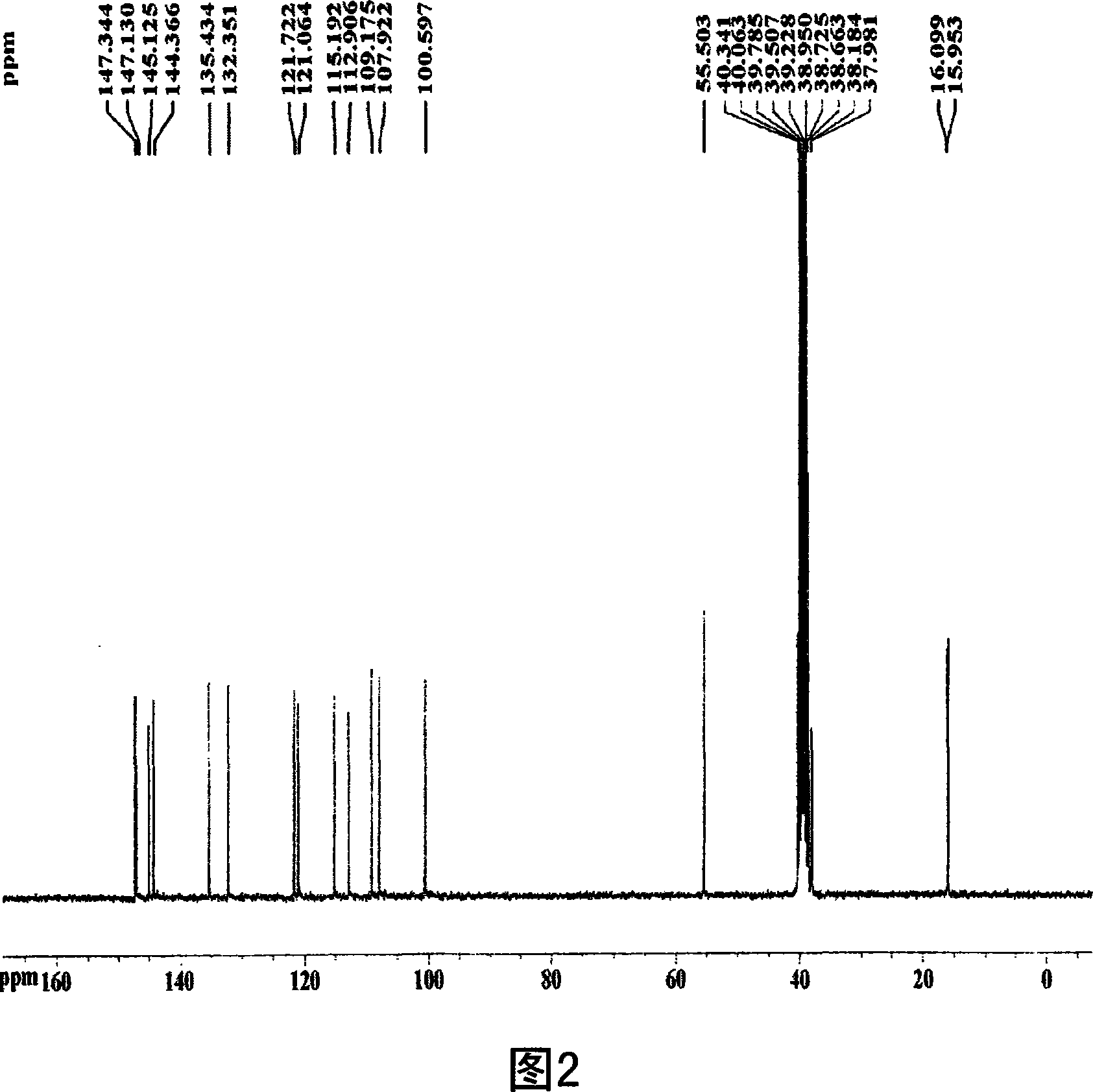
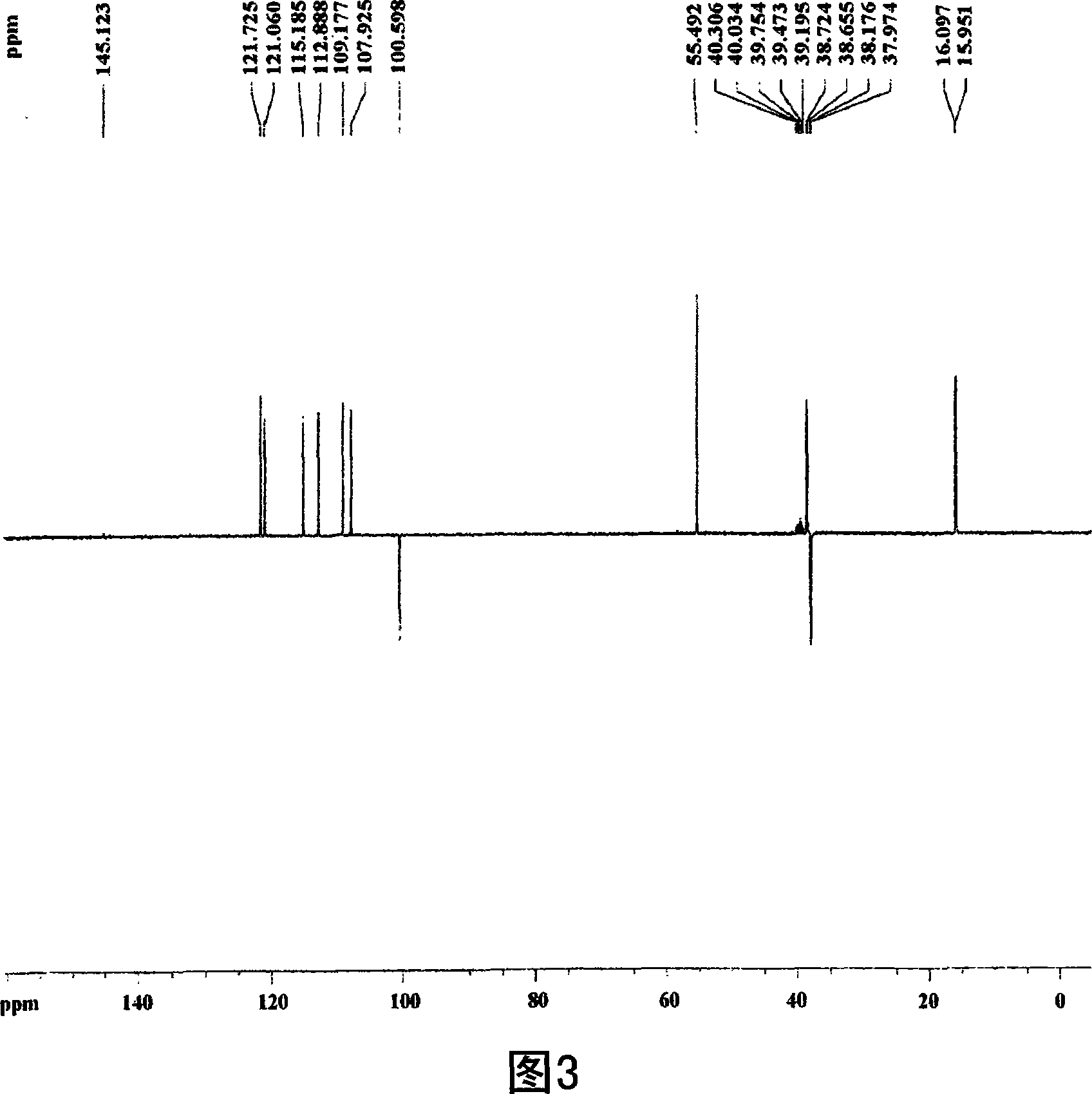
[0074] 75% by volume, 400 ml of methanol was added to 100 g (dry weight) of the dried and pulverized kernels, and the mixture was left to stand at room temperature for 2 days. The above solution was filtered using Whatman No. 2 filter paper. Repeat the above process 2 times. The methanol filtrate was concentrated in vacuo and lyophilized to obtain a crude methanol extract of nucleolus (7 g). The methanol crude extract was fractionated sequentially with ethyl acetate, butanol, and water to obtain ethyl acetate fractions (4.2 g). Utilize silica gel column chromatography (Merck Kieselgel 66; 70-230 order), use hexane and ethyl acetate in the ratio of 10:1 (v / v) the solvent eluting ethyl acetate fractionation, obtains fractionation III ( 1.0g). Using a vacuum rotary concentrator, the solvent was completely removed to obtain a crude extract of the nucleolus. Next, usin...
Embodiment 2
[0084] Investigation of the cytotoxic effect of the lignan compound of the present invention
[0085] Cultivation of RAW264.7 cell line
[0086] In order to investigate the effect of macelignan obtained in Example 1 above on the production of inflammatory response mediators, macrophage RAW264.7 cells were used. Macrophage RAW264.7 cells were purchased from American Tissue Culture Collection (ATCC TIB TIB-71, Rockville, MD, USA). On the DMEM (Dulbecco's Modified Eagle's Medium, Gibco, USA) medium supplemented with heat-inactivated 10% FBS (fetal bovine serum, Gibco, USA), 100 U / ml penicillin G and 100 μm / ml streptomycin, in the medium containing 5 %CO 2 Culture the above cell lines in a 37 °C incubator.
[0087] Determination of Cytotoxicity
[0088] In order to investigate the effect of macelignan of the present invention on the survival rate of RAW264.7 cells, the analysis was based on the reduction of MTT to a purple formazan product by mitochondrial dehydratase (Hayon ...
Embodiment 3
[0091] Investigation of the NO inhibitory effect of the lignan compound of the present invention
[0092] Inhibition of NO production
[0093] Macrophages stimulated by IFN-γ or LPS highly express iNOS and produce a large amount of NO as a mediator of inflammatory response (Miyasaka and Hirata., Immunol. Today., 16:128-130, 1995; Guzik et al., J Physiol. Pharmacol., 54(4):469-487, 2003). Therefore, the effect of macelignan of the present invention on the production of NO in RAW264.7 cells activated by LPS was investigated.
[0094] Dilute RAW264.7 cells to 1 x 10 6 Cells / ml concentration, inoculated into RPMI 1640 medium. After 5 hours, macelignan of the present invention was added at each concentration of 1 to 20 μM, followed by incubation for 2 hours. Next, the medium was treated with LPS (10 µg / ml), and cultured for 24 hours. The control group was treated with LPS only. The amount of NO produced is determined by measuring the NO reaction product NO by using cell cu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com