Optimized targeted MD-2 efficient anti-inflammatory polypeptide
A MD-2, targeted technology, applied in the field of biomedicine, to achieve high affinity, strong anti-inflammatory effect, and reduce the effect of LPS stimulating macrophages to synthesize and release inflammatory mediators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, the construction of phage mutant peptide library
[0037] 1.1 Basic primers and mutation primers
[0038] Basic primers:
[0039] 96Etp:CATGCCCGGGTACCTTTTCTATTCTCACTCT (for construction and colony PCR identification)
[0040] T6:TTTCGGCCGAACCTCCACCATGATTATCCGGCACCGTCTTAGAGTGAGAATAGAAAGGT
[0041] T11: TTTCGGCCGAACCTCCACCATTCCTAGTCAACGGAGAATCAGAGTGAGAATAGAAAGGT
[0042] 96gIIIsp: CCCTCATAGTTAGCGTAACG (for sequencing and colony PCR identification)
[0043] Synthesis strategy for mutant primers:
[0044] The T6 and T11 binding peptides are screened against the huMD2 analog short peptide (FSKGKYKCV). The sequence is located at one end of the β-fold chain of the huMD2 protein and the adjacent random coil region, and is basically linearly distributed. In order to obtain a polypeptide with higher binding capacity, based on the T6 and T11 sequences, the adjacent 3-5 amino acids are randomly mutated each time, the original 2-4 amino acids are retained, and at ...
Embodiment 2
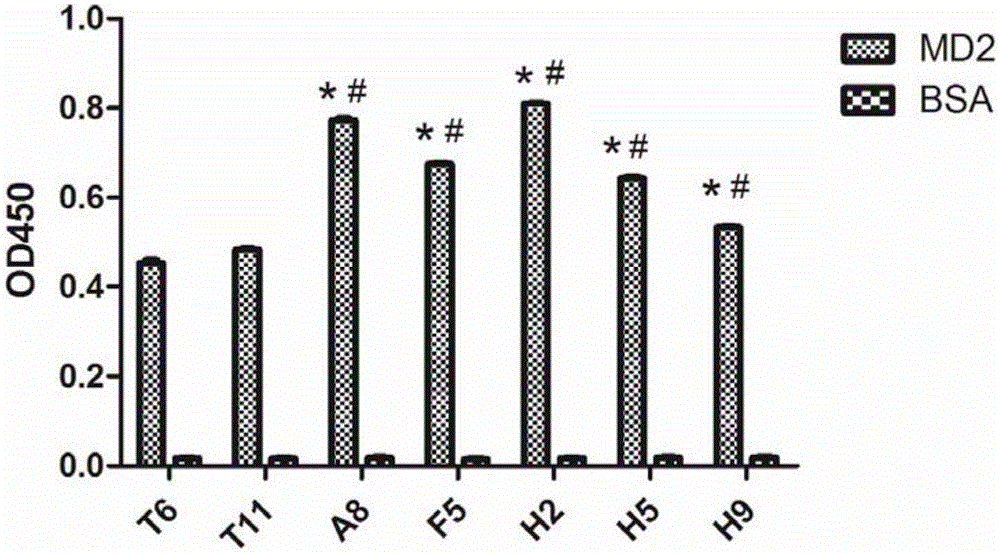
[0081] Example 2, Screening of phage peptide library
[0082] 2.1 Enrichment of specific phage clones with binding activity to MD2 protein
[0083] Coating of immunotubes: 10 μg of antigen huMD2 was diluted to 1 ml with sterile TBS buffer, and immunotubes (Nunc, MaxiSorp) were coated overnight at 4°C. Immunotube sealing: Seal the immunotube with 5ml of 1% BSA blocking solution, and incubate at 37°C for 2h. Washing: discard the blocking solution, and wash the immunotube 3 times with TBST (T: 0.1% Tween-20). Phage binding: add 3ml of phage sample (containing 0.5% BSA and 0.1% Tween-20), incubate at 37°C for 2h, and mix well every 30 minutes. Washing: Wash the immunotube 6 times with TBST (T: 0.1% Tween-20) to wash away unbound phage. Elution: Elute the bound phage with Glycine-HCl (pH 2.1), 1 mL / time, 5 min each time, and then add 160 μl of Tris neutralizing solution. Repeat elution once, then pool. Infection: Take 10 μl from the eluted phage sample for serial dilution and ...
Embodiment 3
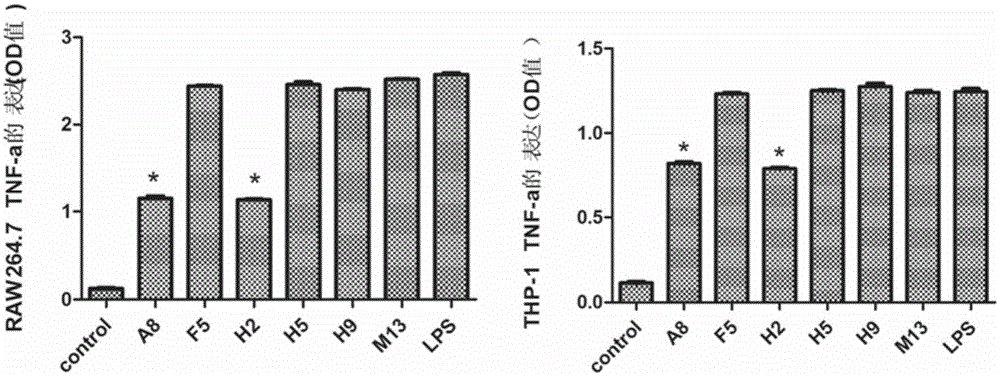
[0104] Embodiment 3, cytokine production inhibition test
[0105] 3.1 Cell Culture
[0106] RAW264.7 was purchased from ATCC, and human promonocytes (THP-1) were donated by the Fourth Laboratory of Field Surgery Institute, Third Military Medical University. RAW264.7 cells and THP-1 cells were cultured in RPMI1640 complete medium containing 10% fetal bovine serum, and THP-1 cells could differentiate into monocyte-macrophage-like cells only after being stimulated by phorbol ethyl ester (PMA) . After THP-1 was cultured and counted, PMA100ng / ml was added for co-cultivation for 24h, and the cell count was adjusted to 1×10 5 pcs / hole. RAW264.7 directly adjusts the cell count to 1×10 5 pcs / hole.
[0107] 3.2 Determination of the inhibitory effect of the 5 clones from the preliminary screening on the cytokine production of RAW264.7 and THP-1
[0108] RAW264.7 and THP-1 induced by PMA were washed 3 times with PBS, and the cell concentration was adjusted to 1×10 with 1640 culture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com