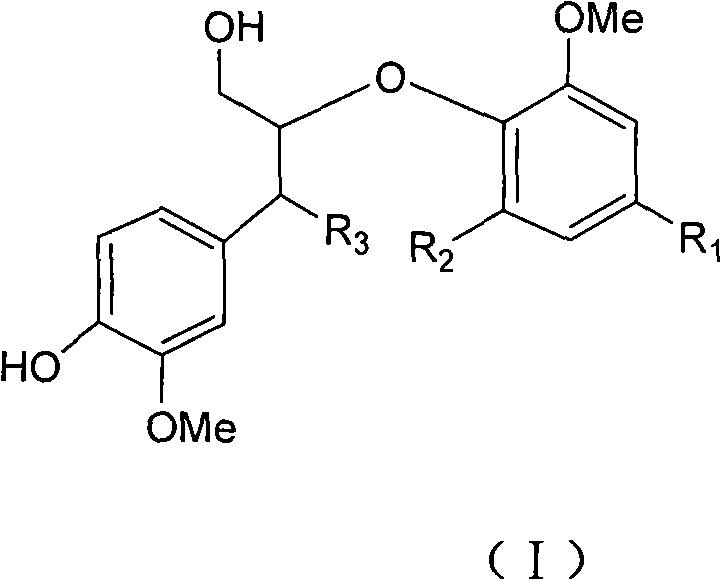
Use of 8-0-4'type lignan in preparing anticomplement medicament
A technology of lignans and anti-complement, which is applied in drug combinations, active ingredients of heterocyclic compounds, allergic diseases, etc., and can solve problems such as complement inhibitory drugs without anti-complement effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
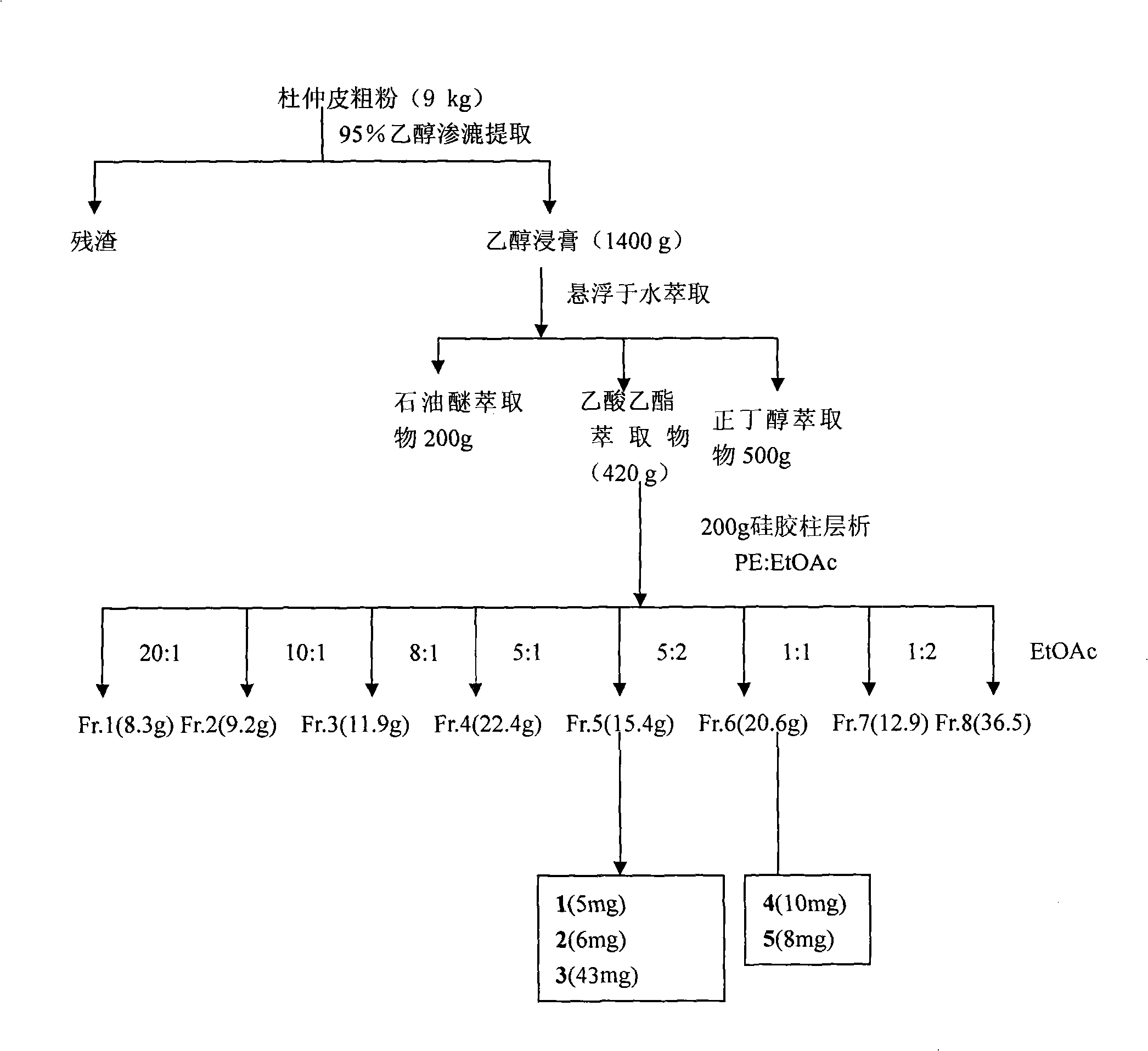
[0022] Embodiment 1 prepares 8-O-4 ' type new lignans
[0023] 9 kg of bark of Eucommia was pulverized, soaked in 95% ethanol (50 L × 3), filtered, combined the filtrates, recovered ethanol under reduced pressure until it had no alcohol smell, and obtained 1.4 kg of ethanol extract. Suspend the ethanol extract with water (3000ml), and extract three times with equal volumes of petroleum ether, ethyl acetate, and n-butanol to obtain petroleum ether fraction (200g), ethyl acetate fraction (420g) and n-butanol fraction (600g) . The three parts were tested for activity, and it was found that the ethyl acetate part had the strongest activity. The ethyl acetate part (200g) was mixed with petroleum ether-ethyl acetate (20:1, 10:1, 8:1, 5:1) successively. , 5:2, 1:1, 1:2, ethyl acetate) gradient elution to obtain 8 fractions (I-VIII). Fraction V (15.4 g) was eluted with petroleum ether / acetone (5:1, 4:1, 2:1, 1:1) to obtain four fractions: Fr.5A-Fr.5D; Silica gel preparation thin la...
Embodiment 2
[0029] Example 2 classical pathway complement inhibition test
[0030] Take guinea pig serum to VBS 2+ Buffer (barbital buffer, pH=7.4, containing 0.5mM Mg 2+ and 0.15mM Ca 2+ ) was diluted 1:80 as a source of complement for the classical pathway. Rabbit anti-sheep erythrocyte antibody in VBS 2+ The buffer was diluted 1:1000 as hemolysin; sheep red blood cells (SRBC) preserved in Alsever's solution were prepared as 2% SRBC. Accurately weigh 1mg of the sample, add VBS 2+ The buffer was dissolved (adding 1% DMSO to aid dissolution), and diluted to 8 concentrations. 200 μl of sample solutions with different concentrations and 200 μl of 1:80 complement were pre-incubated at 37°C for 10 minutes, then 100 μl of hemolysin (1:1000) and 100 μl of 2% SRBC were added in turn, placed in a low-temperature high-speed centrifuge after 30 minutes in a water bath at 37°C, Centrifuge at 5000rpm, 4°C for 10min. Take 200 μl of the supernatant from each tube and place it in a 96-well plate,...
Embodiment 3
[0031] Example 3 Alternative Pathway Complement Inhibition Test
[0032] Serum was taken from healthy adult male volunteers and mixed with VBS-Mg-EGTA buffer (barbital buffer, pH=7.4, containing 5mM Mg 2+ and 8mM EGTA) diluted 1:10, as a source of complement for the alternative pathway. Rabbit red blood cells stored in 3.8% sodium citrate solution were prepared into 2% rabbit red blood cells with VBS-Mg-EGTA buffer solution. Accurately weigh 1 mg of each sample, add VBS-Mg-EGTA buffer solution (1% DMSO is added to aid dissolution), and dilute to 8 concentrations. 150 μl of sample solutions with different concentrations and 150 μl of 1:10 complement were pre-incubated at 37°C for 10 minutes, then 200 μl of 2% rabbit red blood cells were added, placed in a low-temperature high-speed centrifuge after 30 minutes in a water bath at 37°C, and centrifuged at 5000 rpm and 4°C for 10 minutes . Take 200 μl of the supernatant from each tube and place it in a 96-well plate, and measure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com