Caulis trachelospermi total lignans extractive, extraction method and medicine use of the extractive and active constituent thereof
A technology of total lignans and extracts, applied in the field of total lignans and its extraction, can solve the problems of unclear anti-inflammatory and analgesic active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of total lignans extract from vine vine
[0031] Preparation
[0032] Caterpillaria chinensis (1kg) was reflux extracted three times with 95% ethanol (V:W=6:1), filtered through gauze, and concentrated under reduced pressure to obtain 187.5 g of the extract. Dissolve in 95% ethanol, add 282g of diatomite (crude paste: diatomite=1:1.5), mix well, evaporate to dryness, pulverize, add 536ml of petroleum ether to extract three times at room temperature, filter, and concentrate the filtrate to 9.177g of petroleum ether dissolved part . The filter residue was extracted twice with 95% ethanol under reflux. Suction filtration while hot, and the filtrate was concentrated to obtain 173.1 g of extract. Dissolve in 2L of 10% ethanol, centrifuge (3000 rpm, 10 min), take the supernatant and put it on an AB-8 macroporous resin column (column bed volume 2L, pre-equilibrated with 10% ethanol). First elute with 4L of 10% ethanol, and discard the eluate after recovering th...
Embodiment 2
[0034] Anti-inflammatory evaluation of the extract of Example 1 and its main active ingredients
[0035] Experimental Materials
[0036] Experimental animals: male Kunming mice, weighing 18-22 g; male Sprague-Dawley rats, weighing 140-180 g; all were purchased from and bred in the Experimental Animal Center of our hospital.
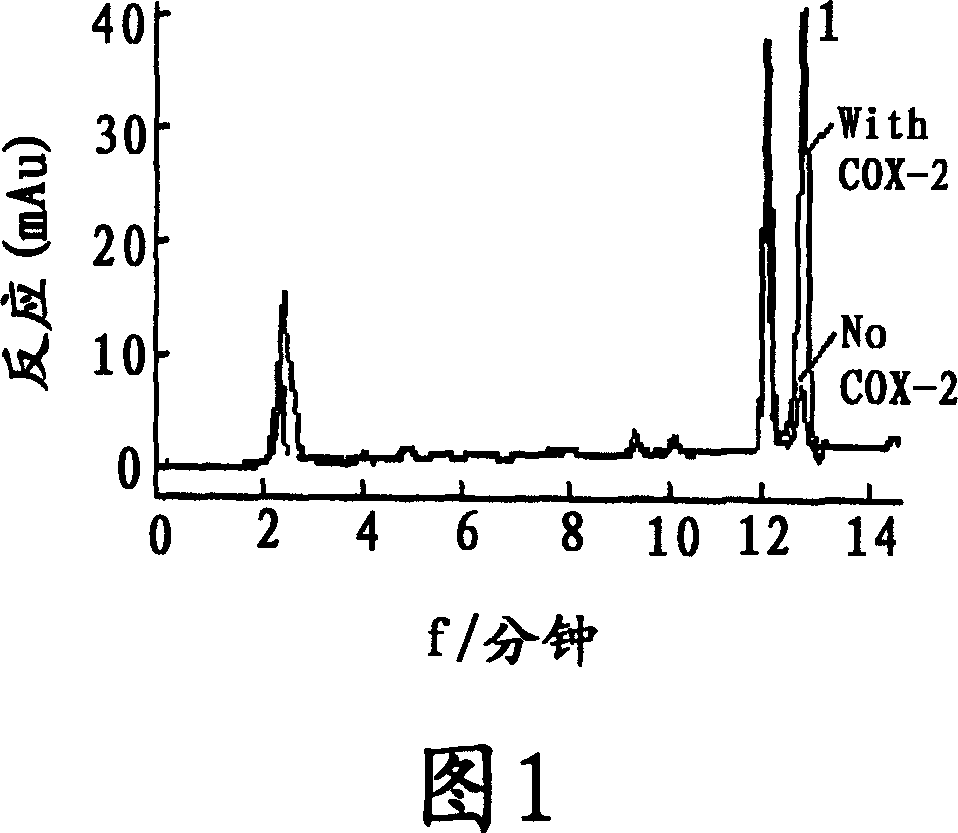
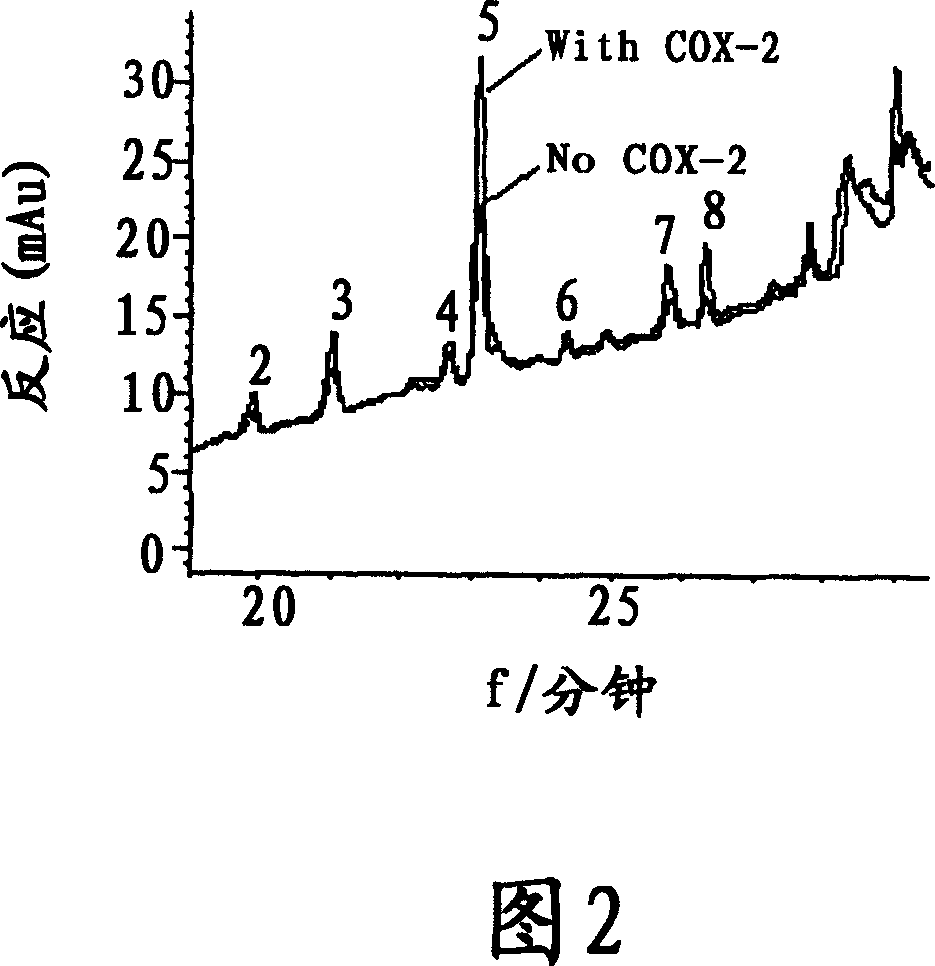
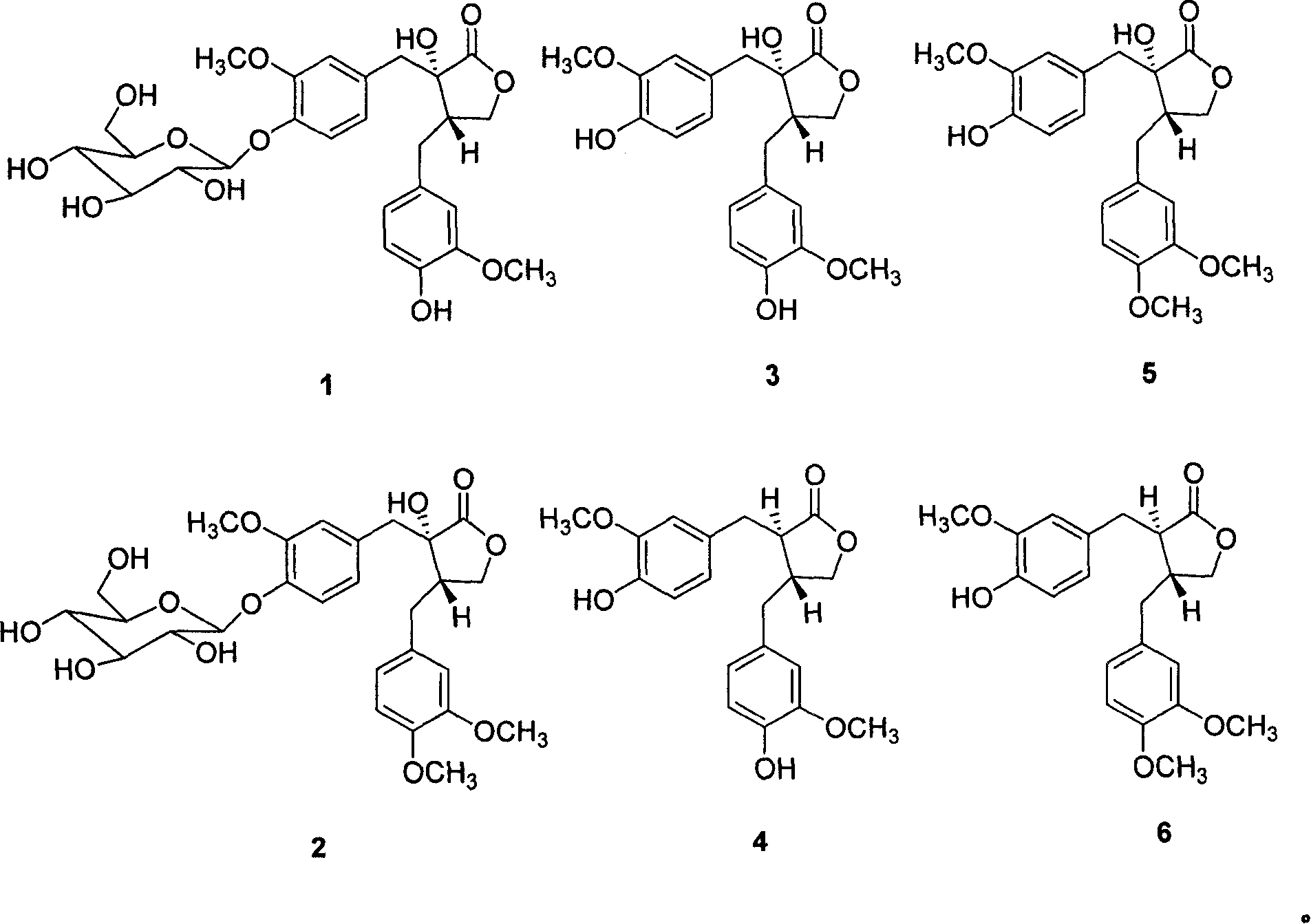
[0037] Tested sample: extract of Example 1; demethylorthoside (1), methanoside (2), demethylosiderin (3), podocarpus resinol (4), terrosidegenin (5) and Arctigenin (6) (separated by the inventors from the extract of Tractoria vine, and the purity measured by liquid phase normalization method is above 98%), celecoxib (manufactured by Pfizer).
[0038] Experimental equipment: hole punch, self-made equipment for measuring rat paw volume, etc.
[0039] Experimental reagents: croton oil and carrageenan were purchased from sigma company, and the others were of domestic analytical grade.
[0040] Experimental method and results:
[0041] Croton oil-induced ear...
Embodiment 3
[0057] Evaluation of the analgesic effect of the extract of Example 1
[0058] Experimental Materials
[0059] Experimental animals: Kunming mice, half male and half male, weighing 18-22 g, purchased from and bred in the Experimental Animal Center of our hospital.
[0060] Test samples: the extract of Example 1, indomethacin (purchased from Cayman Company).
[0061] Experimental equipment: 2L beaker, video camera, etc.
[0062] Reagents used in experiments: all domestic analytical pure.
[0063] Experimental method and results:
[0064] 40 mice were randomly divided into 5 groups, 8 in each group, administered by intragastric administration (ig) (fasting for 16 hours before administration) or intraperitoneal injection (ip) of 0.1ml, and the blank control group was given the same volume of water or normal saline. Half an hour later, intraperitoneally inject (ip) 0.2ml of 0.6% acetic acid solution, and after standing for 5 minutes, record the number of writhing times of eac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com