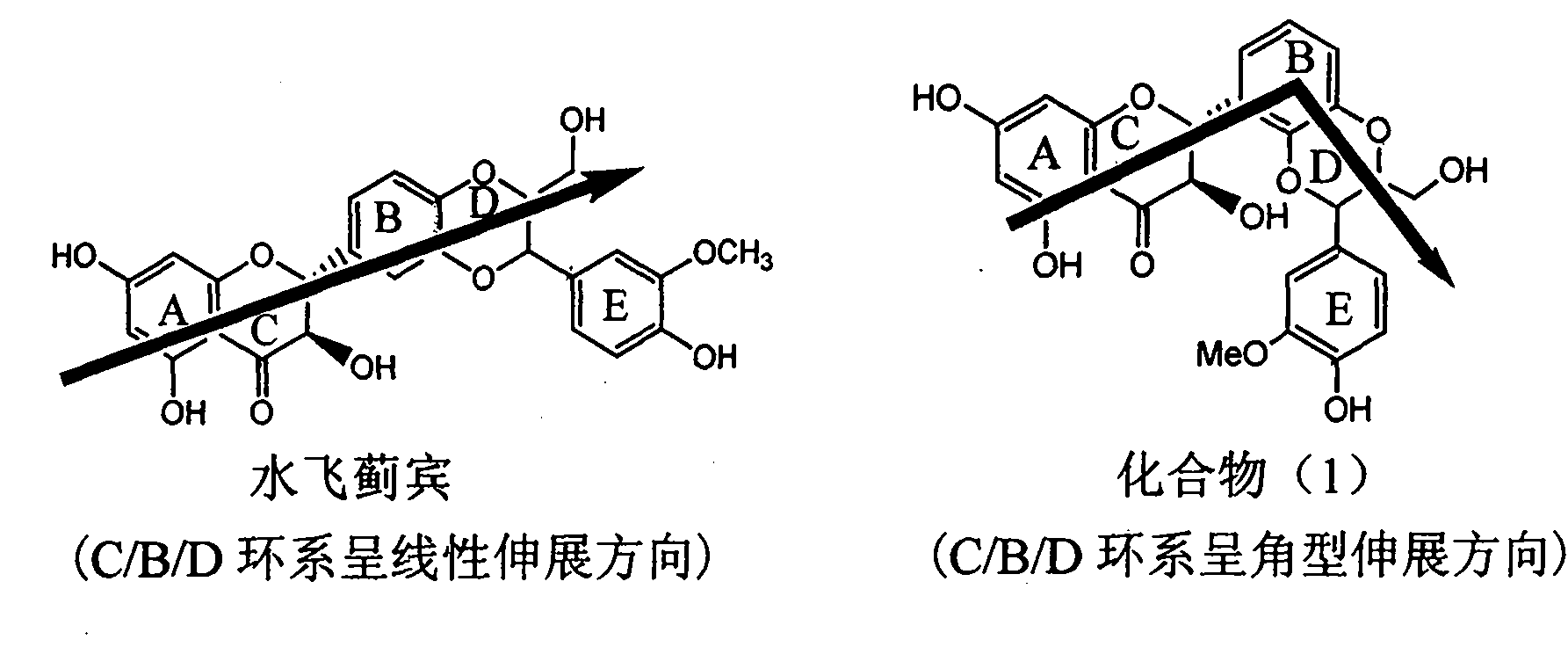
Application of angle flavonoids lignan to preparation of medicaments for treating viral hepatitis B
A flavonoid lignan and horn-shaped technology, which is applied in the field of medicine, can solve problems such as not being effectively developed, and achieve the effects of large-scale production, huge social and economic benefits, and low pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
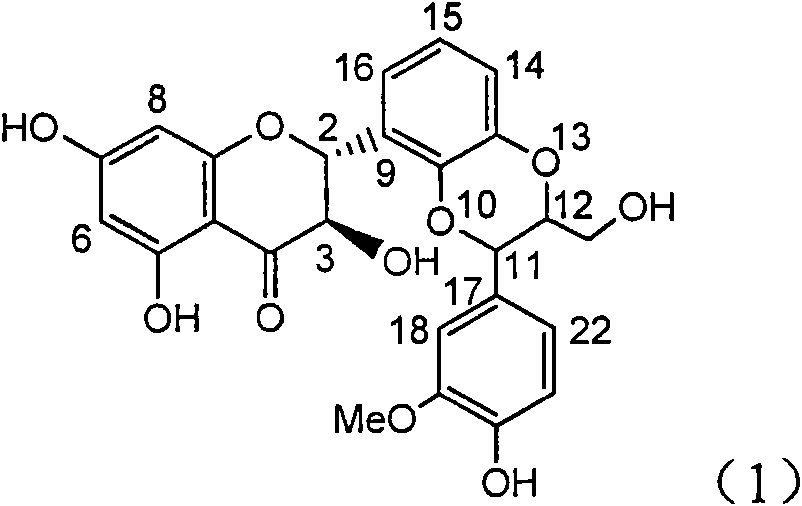
[0024] Example 1: Formula (1) compound (±)-2-[2,3-dihydro-3-(3-methoxy-4-hydroxyphenyl)-2-hydroxymethyl-1,4 benzodioxane -5] Preparation of -2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one
[0025] Instruments and reagents:
[0026] The ultraviolet spectrum was measured with a Shimadzu UV-240 ultraviolet spectrophotometer; the hydrogen nuclear magnetic resonance spectrum 1 H-NMR is measured by INOVA type superconducting nuclear magnetic resonance spectrometer (VARIAN INOVA-400MHz) (tetramethylsilyl ether TMS is the internal standard); (100-200, 200-300 and 300-400 mesh) and silica gel GF254 (10-40 mesh) for thin layer chromatography are all produced by Qingdao Ocean Chemical Factory; all reagents used are analytically pure, and the boiling range of petroleum ether is 60 -90°C; thin-layer preparative chromatography (PTLC) uses aluminum foil silica gel plates from Merck; column chromatography uses dextran gel SephadexLH-20 from Amersham Pharmacia Biotech AB in Sweden; rev...
Embodiment 2
[0045] Example 2: Inhibition of the replication of hepatitis B virus deoxyribonucleic acid (HBV DNA) secreted by the compound of formula (1) to HepG2.2.15 cells
[0046] 2.1 Cell culture:
[0047] HepG2.2.15 cells were cultured in DMEM medium containing 10% inactivated fetal bovine serum, 100 U / ml penicillin and 100 U / ml streptomycin, 100 μg / ml G418 at 37°C, 5% CO 2 , cultured in an incubator with 100% relative humidity.
[0048] 2.2 The inhibitory effect of compound (1) on the growth of HepG2.2.15 cells was determined by MTT method:
[0049] Take the HepG2.2.15 cells in the logarithmic growth phase, and dilute the cells to 1×10 with medium 5 cells / ml, seeded in 96-well cell culture plate, 100 μl per well, at 37°C, 5% CO 2 , add compound (1) diluted with culture medium after 24 hours in the incubator of 100% relative humidity, the concentration is respectively 1000 microgram / ml, 200 microgram / ml, 40 microgram / ml, 8 microgram / ml, every well microliter, each concentration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com