A kind of anti-hepatic fibrosis composition and its application
A liver fibrosis and compound technology, applied in the field of medicine, can solve problems such as gastrointestinal reactions, achieve clear mechanism, enhance therapeutic effect, and reduce the degree of liver fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Silybin and Leoceque compositions for the treatment of liver fibrosis model mice
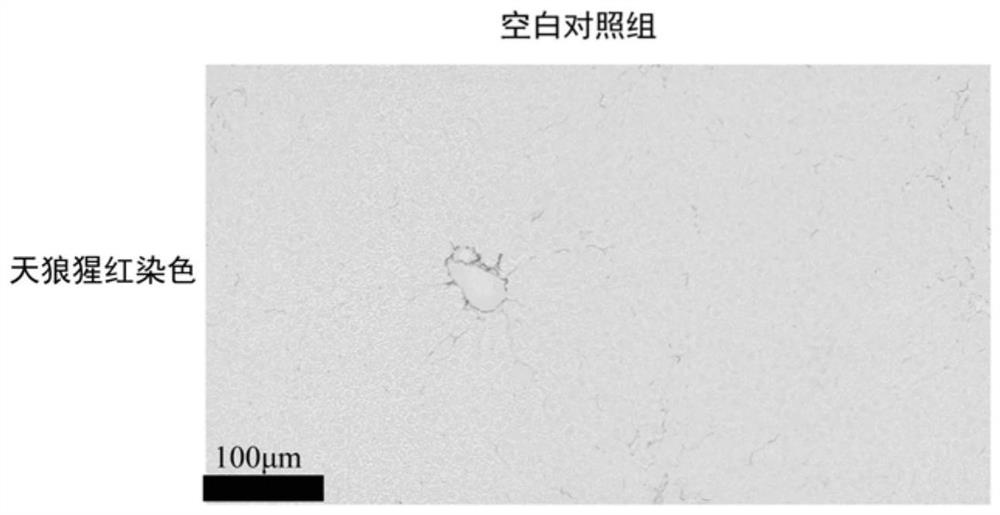
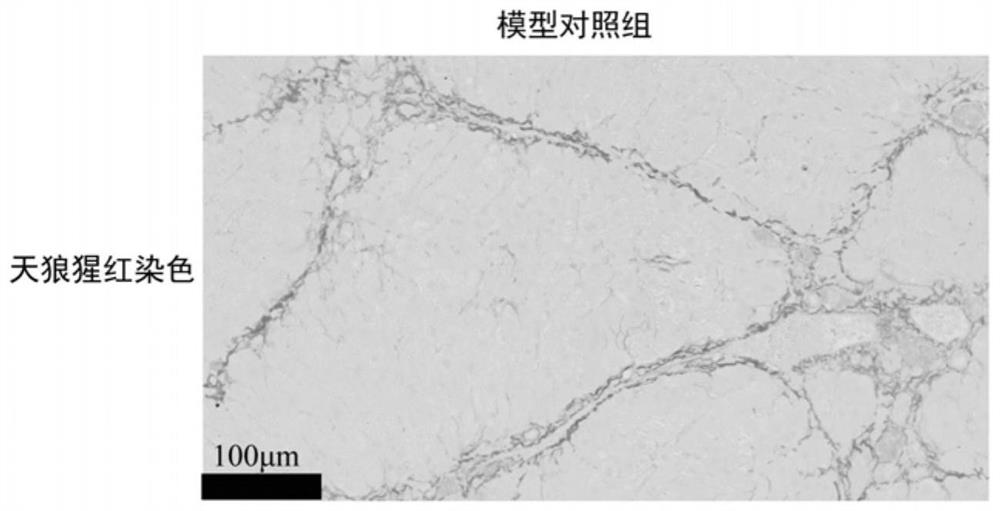
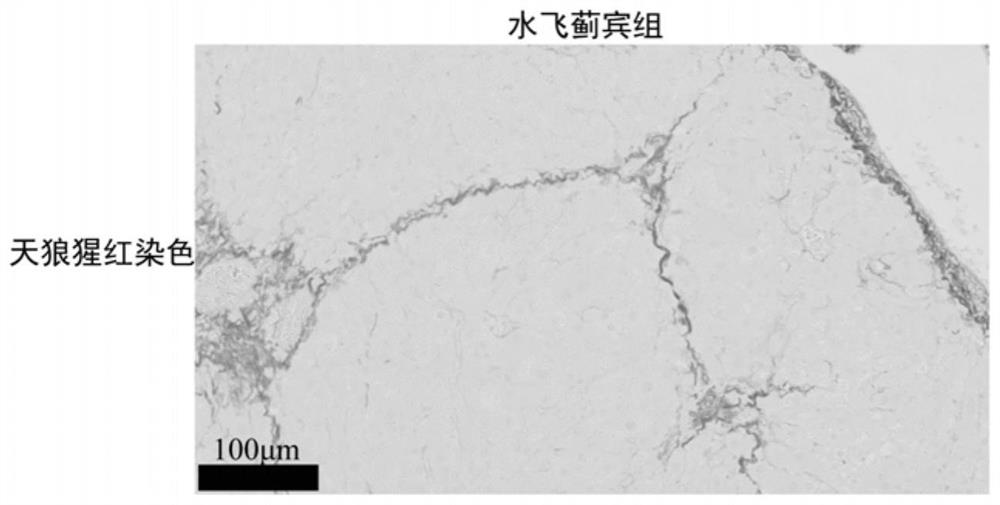
[0030]In addition to the normal control group, the mice with successful molding were randomly divided into model control group (Model-control), silybin group (6mg / kg, iv), Leocerin group (1mg / kg, iv), silybin and Leoceque composition group, each experimental group of 6 mice, given once a day, administered for 4 weeks, a total of 28 times, and recorded mouse weight changes during the experiment. The dosage administered in the composition group is consistent with the dose of each drug in the monotherapy group. All groups of mice were administered until the last day of the experiment, weighed 12 h in advance and fasted and did not tolerate water. After peeling off the large lobes of the liver, wash them with pre-chilled normal saline, take roughly the same parts in 4% paraformaldehyde solution and fix them, and then perform embedding, sectioning and Sirius red staining.
[0031] as Figure 1-5 As...
Embodiment 2
[0033] Silybin and Leocetin anti-hepatic fibrosis dose screening study
[0034] The molded animals after 4 weeks of liver fibrosis molding were randomly divided into four groups, the module group, silybin 6mg / kg + leocerin 0.5mg / kg group, silybin 6mg / kg + leosixin 1mg / kg group, silybin 1mg / kg + leosique 1mg / kg group, each group of 6 mice, respectively, the concentration of silybin 6mg / kg + liosic 0.5mg / kg, silybin 1mg / kg + liosicin 1mg / kg, silybin 6mg / kg, silybin 6mg / kg respectively, respectively, the concentration of silybin 6mg / kg + silybin 0.5mg / kg, silybin 1mg / kg, silybin 6mg / kg Kg + Leocet 1 mg / kg three groups of suspension drug solution, tail vein administration, every 3 days to administer once, a total of 10 times. After the end of administration, the liver lobe was peeled off and washed with pre-cooled normal saline, and after fixation of roughly the same part in 4% paraformaldehyde solution, it was embedded, sliced and stained with Sirius red, and positive area analysi...
Embodiment 3
[0037] Drug carrier-loaded silybin and leoceque compositions for the treatment of liver fibrosis
[0038] Preparation of PAMAM micelles of silybin: weigh silybin 6mg, add 0.6mL of methanol, ultrasonically dissolve to make a methanol solution of silybin, dissolve PAMAM 15mg in 1.5mL of double distilled water to form a carrier solution, add the methanol solution of silybin to the carrier solution dropwise, and stir for 3h after the completion of the drops. Dialysis in a dialysis bag (MWCO= 1000) for 24h, 3000 rpm centrifugation for 10min, take the supernatant lyophilized, that is, the PAMAM micelle of silybin.
[0039] PAMAM micelle preparation of Liosicine: According to the PAMAM micelle preparation method of silybin, replace "silybin 6mg" with "Leosimine 1mg", that is, The PAMAM micelle of Leosilana.
[0040] MPP micelle preparation of silybin: weigh silybin 6mg, add methanol 0.6mL, ultrasonically dissolve to make a methanol solution of silybin, dissolve mPP 20mg in 2mL double dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com