New medical application of polyethylene glycol loxenatide or pharmaceutical composition thereof
A polyethylene glycol and composition technology, applied in the field of biomedicine, can solve the problems of research on the treatment effect of type II diabetes patients, and the treatment effect of type II diabetes patients without polyethylene glycol loxenatide, and achieve significant myocardial Protective effect, protection of liver damage, effect of alleviating liver fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1. PEG-Loxe improves lipid disorders in T2DM mice
[0045] 1.1 Experimental method
[0046] Blood samples were immediately centrifuged (1200 g, 4°C, 15 min) to obtain serum. The levels of TC, TG, HDL-C, LDL-C, HbA1c and insulin in serum were detected by automatic biochemical analyzer (BS-420, Mindray, China).
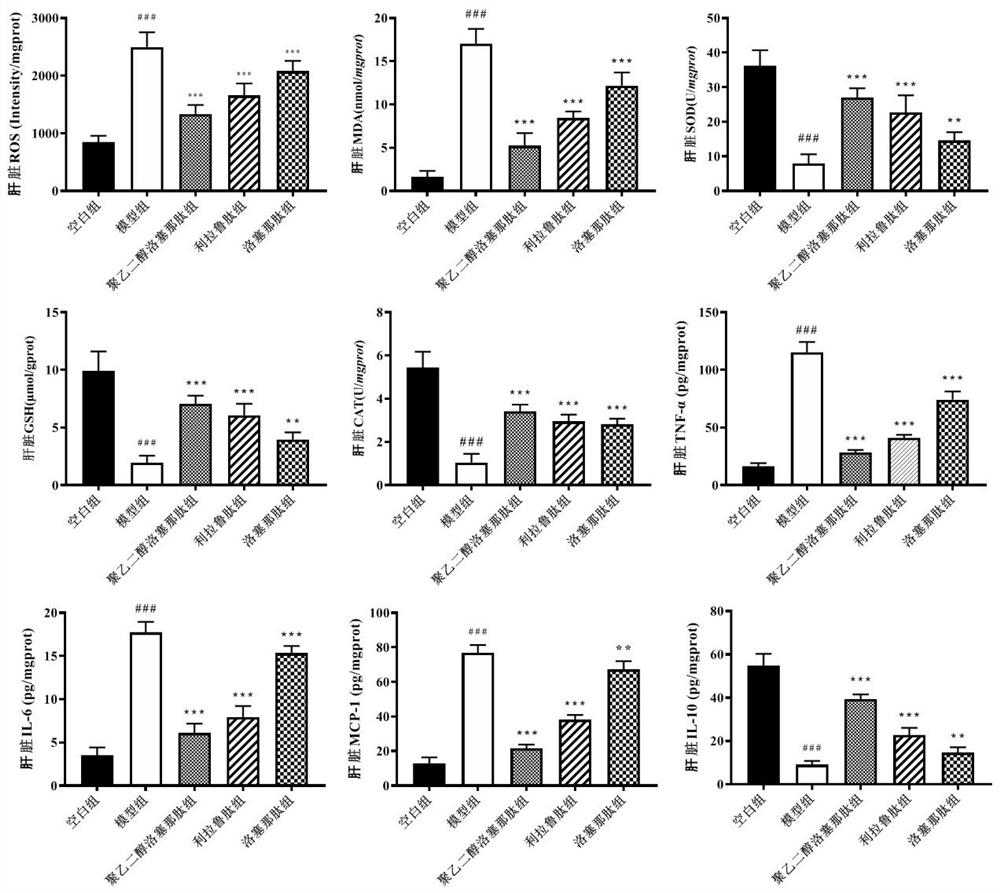
[0047] The liver tissue was homogenized with 9× (wt / vol) ice-cold phosphate-buffered saline, then centrifuged at 3500 rpm for 15 min, and the supernatant was collected. According to the manufacturer's instructions, ELISA kits were used to detect lipids (TC, TG, HDL-C, LDL-C), AST, ALT), oxidative factors (ROS, MDA, SOD, GSH, CAT) and inflammation in liver tissue. Sex factor (IL-10, TNF-α, IL-6, MCP-1) content.
[0048] 1.2 Experimental results
[0049] Table 1. PEG-Loxe can improve the levels of various biochemical parameters in db / db mice
[0050]
[0051] Note: NC is the normal control group; T2DM is the type 2 diabetes group. Data are expresse...
Embodiment 2
[0054] Example 2. PEG-Loxe improves hepatic steatosis and liver injury in T2DM mice
[0055] 1.1 Experimental method
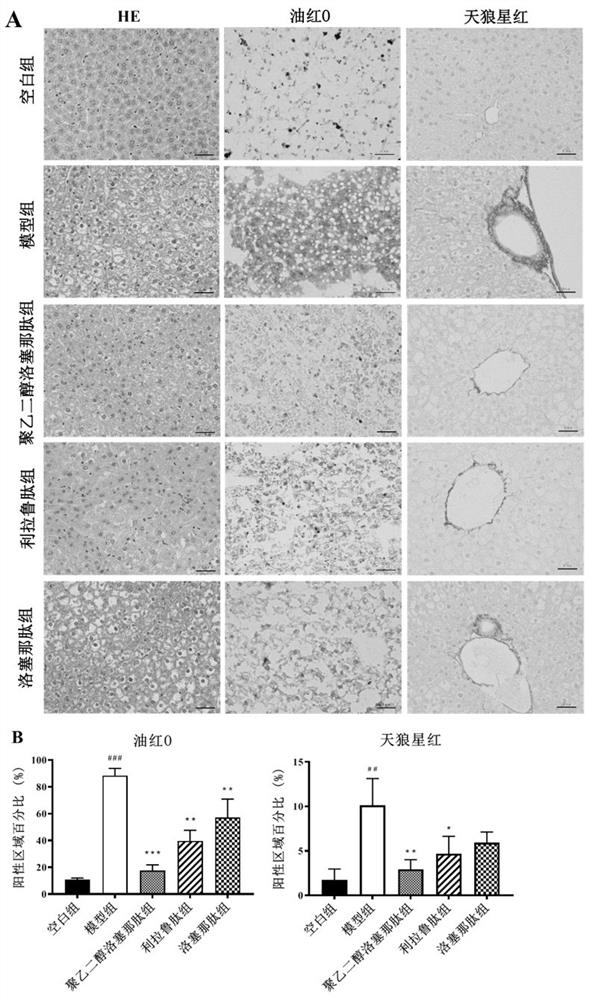
[0056] Animal liver and pancreas tissues were fixed in 10% formalin solution, dehydrated and embedded in paraffin. Embedded sections (3-5 μm thickness) were stained with hematoxylin and eosin (H&E) for histopathological analysis. Liver sections were stained with Sirius Red and Oil Red O, and all sections were observed and captured at 400× using an Olympus BX51 microscope (Tokyo, Japan).
[0057] 1.2 Experimental results
[0058] H&E staining of liver sections showed that the hepatocytes in the blank group were regular in shape, evenly distributed and tightly arranged; cytoplasmic vacuoles and hepatocyte necrosis appeared in T2DM mice. However, after 4 weeks of treatment with PEG-Loxe, Lira, and Loxe, liver lesions were significantly reduced (attached figure 1 A). In order to evaluate the liver damage in T2DM mice, we measured the levels of ALT and AST in ...
Embodiment 3
[0063] Example 3: PEG-Loxe reduces hepatic oxidative stress and inflammatory response in T2DM mice
[0064] 1.1 Experimental method
[0065] The liver tissue was homogenized with 9× (wt / vol) ice-cold phosphate-buffered saline, then centrifuged at 3500 rpm for 15 min, and the supernatant was collected. According to the manufacturer's instructions, ELISA kits were used to detect lipids (TC, TG, HDL-C, LDL-C), AST, ALT), oxidative factors (ROS, MDA, SOD, GSH, CAT) and inflammation in liver tissue. Sex factor (IL-10, TNF-α, IL-6, MCP-1) content.
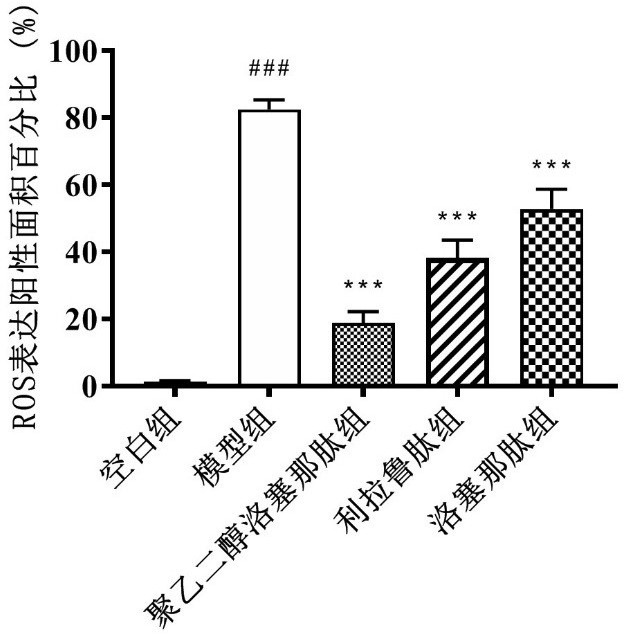
[0066] ROS production in the liver was further detected by DHE staining. Briefly, 10 μm thick frozen liver tissues were obtained for sectioning using a cryostat (CM1900, Leica, Germany). Subsequently, the tissue was incubated with 5 mmol / L fluorescently labeled DHE for 30 min at 37°C in a dark environment, DHE was diluted 1:1000, and then treated with 4',6'-diamino-2-phenylindole (DAPI , AS1075, Aspen Biological, Wuhan, China) staini...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com