Application of B ring methoxy substituted silybin to preparing glycosidase inhibitors
A technology of silibinin ester and methoxy group is applied in the field of pharmaceutical use of B-ring methoxy silibinin for preparing glycosidase inhibitors, which can solve the problems such as diabetes that have not been inhibited by glycosidase, and achieve industrial Clear prospects, convenient sources, huge social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
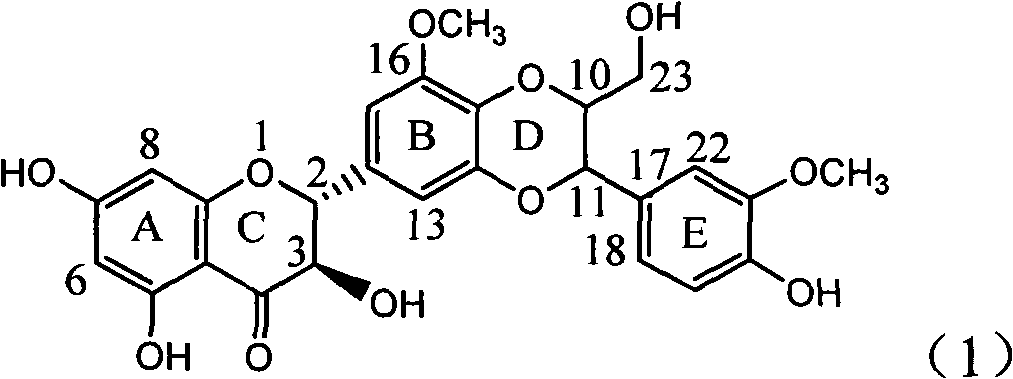
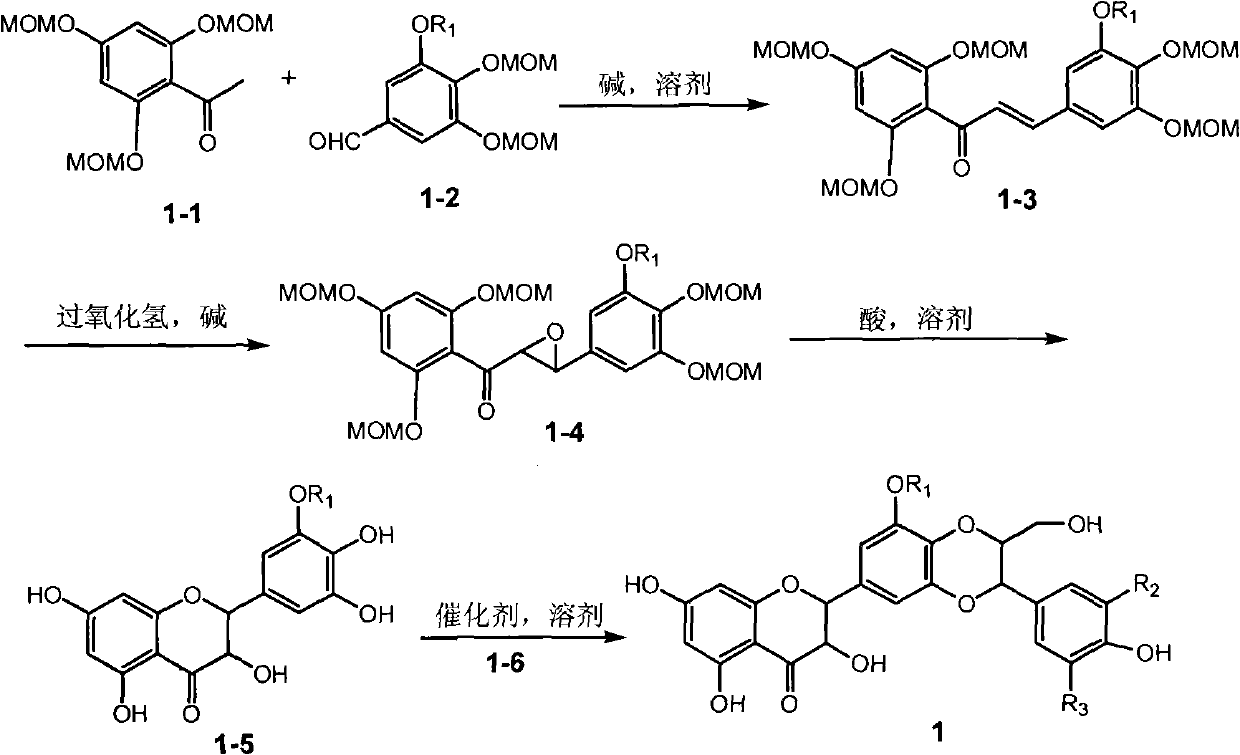
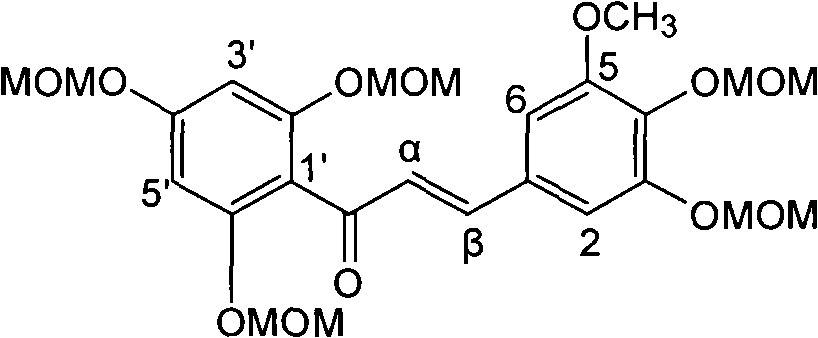
[0021] Example 1: Compound of formula (1) (±)-2-[2,3-dihydro-3-(3-methoxy-4-hydroxyphenyl)-2-hydroxymethyl-8-methoxy-1,4 Preparation of -benzodioxane-6-]-2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one
[0022] 1.1 Instruments and reagents:
[0023] The ultraviolet spectrum was measured with a Shimadzu UV-240 ultraviolet spectrophotometer; the hydrogen nuclear magnetic resonance spectrum 1 H-NMR is measured by INOVA type superconducting nuclear magnetic resonance spectrometer (VARIAN INOVA-400MHz) (tetramethylsilyl ether TMS is the internal standard); (100-200, 200-300 and 300-400 mesh) and silica gel GF254 (10-40 mesh) for thin-layer chromatography are all produced by Qingdao Ocean Chemical Factory; the reagents used are all analytically pure; thin-layer preparative chromatography (PTLC ) uses the aluminum foil silica gel plate of Merck Company; Sephadex LH-20 used for column chromatography adopts the product of Amersham Pharmacia Biotech AB Company of Sweden; Reversed-...
Embodiment 2
[0036] Example 2 : Compound (1) (±)-2-[2,3-dihydro-3-(3-methoxy-4-hydroxyphenyl)-2-hydroxymethyl-8-methoxy-1,4 Inhibitory activity of -benzodioxane-6-]-2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one on α-glucosidase
[0037] 2.1 Instruments and reagents
[0038] 2.1.1 Experimental Instruments
[0039] Microplate reader: ELISA plate reader (Bio-Tek Instruments, USA)
[0040] 2.1.2 Reagents
[0041] α-glucosidase is α-D-glucosidase (Sigma, 500U / ml); 4-nitrophenol-α-D-glucopyranoside (PNPG, Merck), reduced glutathione (Shanghai Shenggong) , Acarbose is Baitangping (Bayer Healthcare Co., Ltd., Beijing).
[0042] 2.2 Test method
[0043] The inhibitory effect of the compound on α-glucosidase is determined by colorimetry: add phosphate buffer (67 mmol / liter, pH 6.8, 170 microliters), reduced glutathione (1 mg / ml, 5 microliter), α-D-glucosidase (diluted to 0.2U / ml with phosphate buffer, 25 microliters), compound (1) was dissolved in dimethyl sulfoxide, diluted with phosph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com