Preparation of flavonolignans in milk thistle and application thereof
A technology of lignans and milk thistle, applied in the field of natural medicinal chemistry, can solve the problems of unreported anti-diabetic activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: Separation, purification and preparation of flavonoid lignans 1-11
[0017] 1 Experimental method
[0018] Use petroleum ether to heat and reflux the pulverized milk thistle seeds for 3 times, each time for 2 hours, to obtain 1.6 L of fatty oil, then continue to heat the residue with 95% ethanol for 3 times, each time for 2.5 hours, and combine the three extractions solution, filtered and then rotary evaporated to obtain 2.0Kg of extract. A part of the extract was taken and prepared at a concentration of 1.0 mg / mL for the PTP1B enzyme inhibitory activity test, and the inhibition rate was as high as 73.1%. The extract was uniformly dispersed with water, and then extracted with equal volumes of dichloromethane, ethyl acetate and n-butanol to obtain a dichloromethane layer, ethyl acetate layer, n-butanol layer and a water layer. Different extraction layer samples were prepared to a concentration of 1.0mg / mL for the PTP1B enzyme inhibition activity test, the in...
Embodiment 2
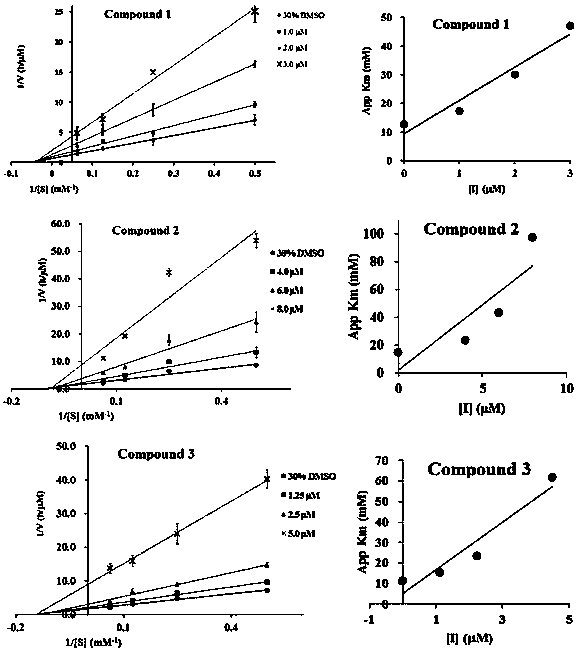
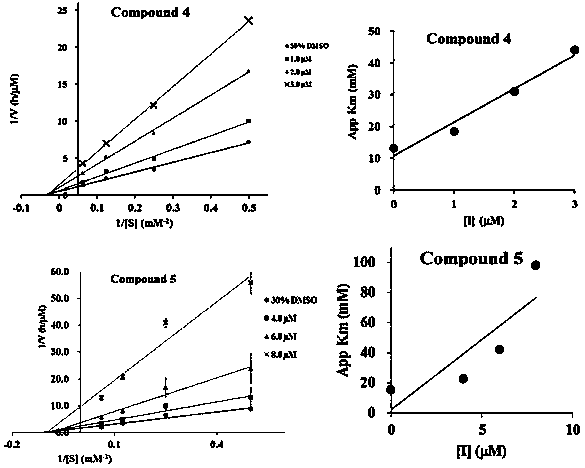
[0021] Example 2: Inhibition of flavonoid lignans to PTP1B, TCPTP, VHR, SHP-1, SHP-2
[0022] 1 Experimental method
[0023] Preparation of PTP1B and VHR buffer: Take 0.06M sodium citrate (pH 6.0), 0.1M sodium chloride, 1mM EDTA, and 1mMDTT to prepare a buffer solution with a pH of 7.
[0024] Preparation of TCPTP, SHP-1, and SHP-2 buffer: Take 25mM Tris / HCl, 50mM NaCl, 2mM EDTA, 5mMDTT, 0.01% Brij35, 1mg / mL bovine serum albumin to prepare a buffer solution with a pH of 7.
[0025] Preparation of positive control and samples to be tested: Dissolve 1.142mg of oleanolic acid in DMSO and dilute to 2.5mL as a stock solution, and then dilute it with buffer solution to form 500, 100, 50, 10μM serial solutions. Take the corresponding mass of the compound to be tested, dissolve it in DMSO to make 1mM stock solution, and then dilute it with buffer solution to make 500, 100, 50, 10, 5μM serial solutions.
[0026] Preparation of PTP1B enzyme: 0.1 μL enzyme was dissolved in 80 μL buffer...
Embodiment 3
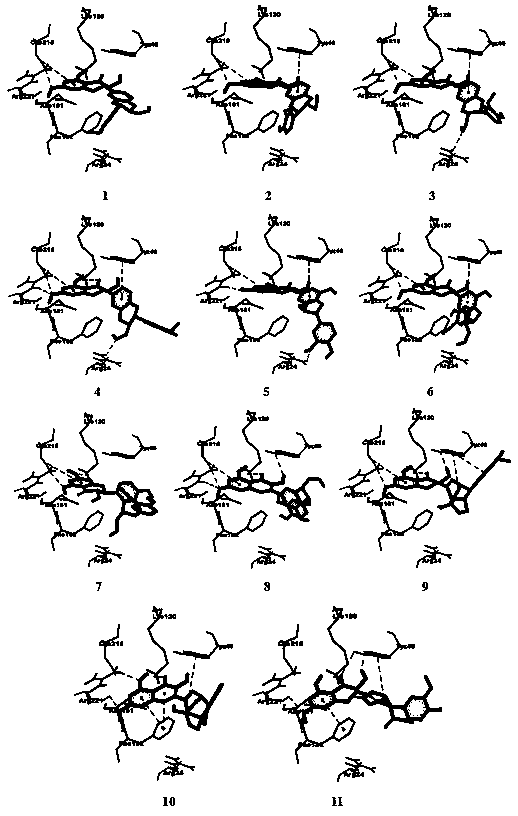
[0031] Example 3: Molecular simulation docking of flavonoid lignans and PTP1B
[0032] 1 Experimental method
[0033] Find the crystal structure of the target protein PTP1B (PDB: 2QBP) through the RCSB protein database (PDB) with a resolution of Prepare protein through software DS 3.0, including hydrogenation, dehydration, loop completion, protein plus polar hydrogen. The flavonoid lignans were prepared by drawing the relevant structures with ChemDraw 14.0, importing them into DS 3.0, and converting them into mol2 structures. And use sybyl 2.0 to optimize the relevant structure, the relevant parameters are as follows: select Tripos force field in the Force Field option menu; select Gasteiger-Huckel charge in the Charges option menu; increase the maximum number of repetitions (Max.Iterations) to 1000; reduce the Gradient to 0.005 ;Small molecule plus all hydrogen and other parameters default.
[0034] Import the prepared protein and flavonoid lignans into Autodock4.2, deter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com