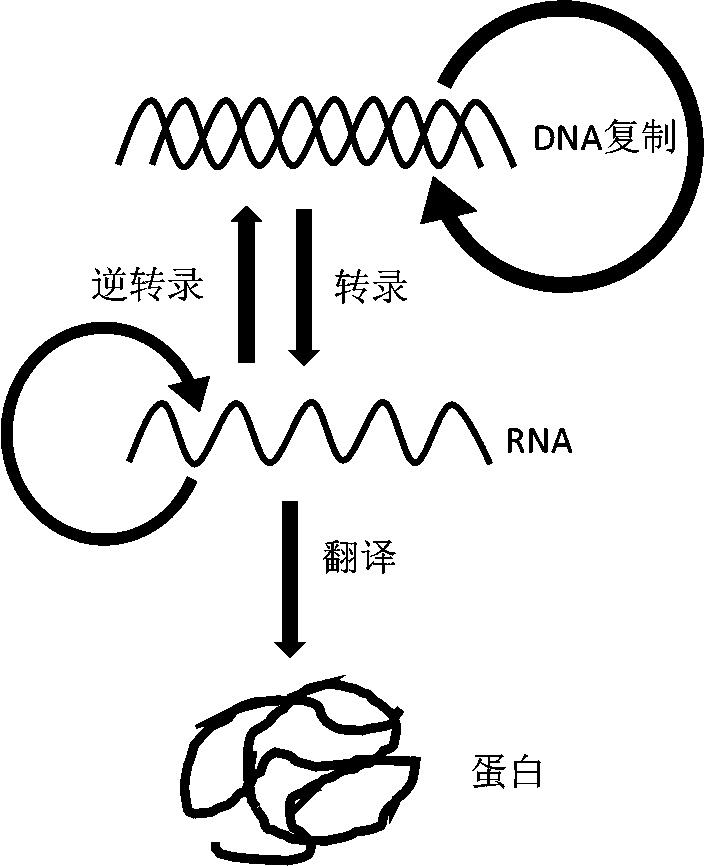
Synthesis system, preparation, kit and preparation method of in-vitro DNA-to-Protein (D2P)
A synthesis system and system technology, applied in the direction of recombinant DNA technology, introduction of foreign genetic material using vectors, fermentation, etc., can solve the limitation of biosynthesis efficiency and yield, complex experimental requirements, increase operation time, manufacturing cost and experimental complexity, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0248] In the present invention, the preparation method of the yeast cell extract is not limited, and a preferred preparation method includes the following steps:
[0249] (i) providing yeast cells;
[0250] (ii) washing the yeast cells to obtain washed yeast cells;
[0251] (iii) subjecting the washed yeast cells to destructive treatment to obtain crude yeast extract;
[0252] (iv) performing solid-liquid separation on the crude yeast extract to obtain the liquid part, which is the yeast cell extract.
[0253] In the present invention, the solid-liquid separation method is not particularly limited, and a preferred method is centrifugation.
[0254] In a preferred embodiment, said centrifugation is performed in a liquid state.
[0255] In the present invention, the centrifugation conditions are not particularly limited, and a preferred centrifugation condition is 5000-100000 g, preferably 8000-30000 g.
[0256] In the present invention, the centrifugation time is not particu...
Embodiment 1
[0287] Example 1: Amplification of plasmid templates using phi29 DNA polymerase
[0288] 1.1 Preparation of DNA amplification system: random primers at a final concentration of 20-30 μM, plasmid template at 0.05-0.15 μg / mL, dNTP at 0.5-1 mM, 2 × BSA, and phi29 DNA aggregation at 0.05-0.1 mg / mL Enzyme, 1 × phi29 reaction buffer (consisting of 50 mM Tris-HCl, 10 mM MgCl 2 , 10 mM (NH 4 ) 2 SO 4 , 4 mM DTT, pH7.5).
[0289] 1.2 Amplification reaction of plasmid template DNA in vitro: Place the above-mentioned reaction system in an environment of 20-30 °C for 6-12 h, usually overnight.
Embodiment 2
[0290] Example 2: In vitro protein synthesis using amplified template DNA
[0291] 2.1 In vitro protein synthesis system: final concentration of 22 mM 4-hydroxyethylpiperazineethanesulfonic acid at pH 7.4, 30-150 mM potassium acetate, 1.0-5.0 mM magnesium acetate, 1.5-4 mM nucleoside triphosphate mixture (adenoside Purine nucleoside triphosphate, guanosine triphosphate, cytidine nucleoside triphosphate and uridine nucleoside triphosphate), 0.08-0.24 mM amino acid mixture (glycine, alanine, valine, leucine, Isoleucine, Phenylalanine, Proline, Tryptophan, Serine, Tyrosine, Cysteine, Methionine, Asparagine, Glutamine, Threonine, Aspartic Acid, Glutamine acid, lysine, arginine, and histidine), 25 mM creatine phosphate, 1.7 mM dithiothreitol, 0.27 mg / mL creatine phosphokinase, 0.027-0.054 mg / mL T7 RNA polymerase, 1% - 4% polyethylene glycol, 0.5% - 2% sucrose, and finally 50% by volume of yeast cell extract;
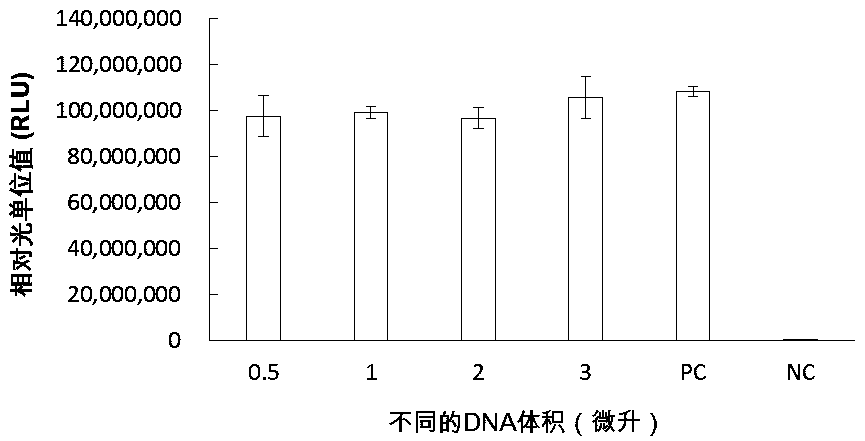
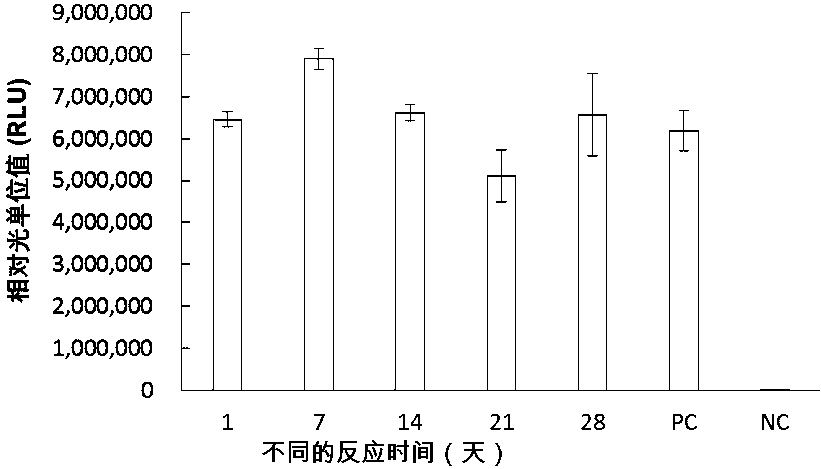
[0292] 2.2 In vitro protein synthesis reaction: Add 0.5-3 μL of amplif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com