Heparin binding protein detection test paper for immunomicrosphere chromatography detection
The technology of heparin-binding protein and immune microspheres is applied in the field of preparation of test strips for detecting heparin-binding protein by immunomicrosphere chromatography, which can solve the problems of no diagnostic reagent method, low accuracy and stability, long time consumption, etc. Achieve the effect of ensuring validity and quality control, clear and accurate test results, and strong repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
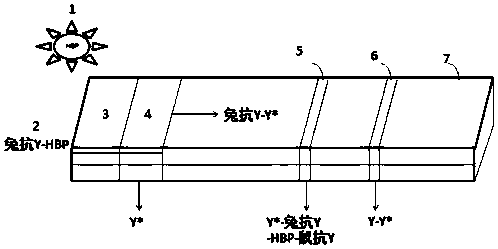
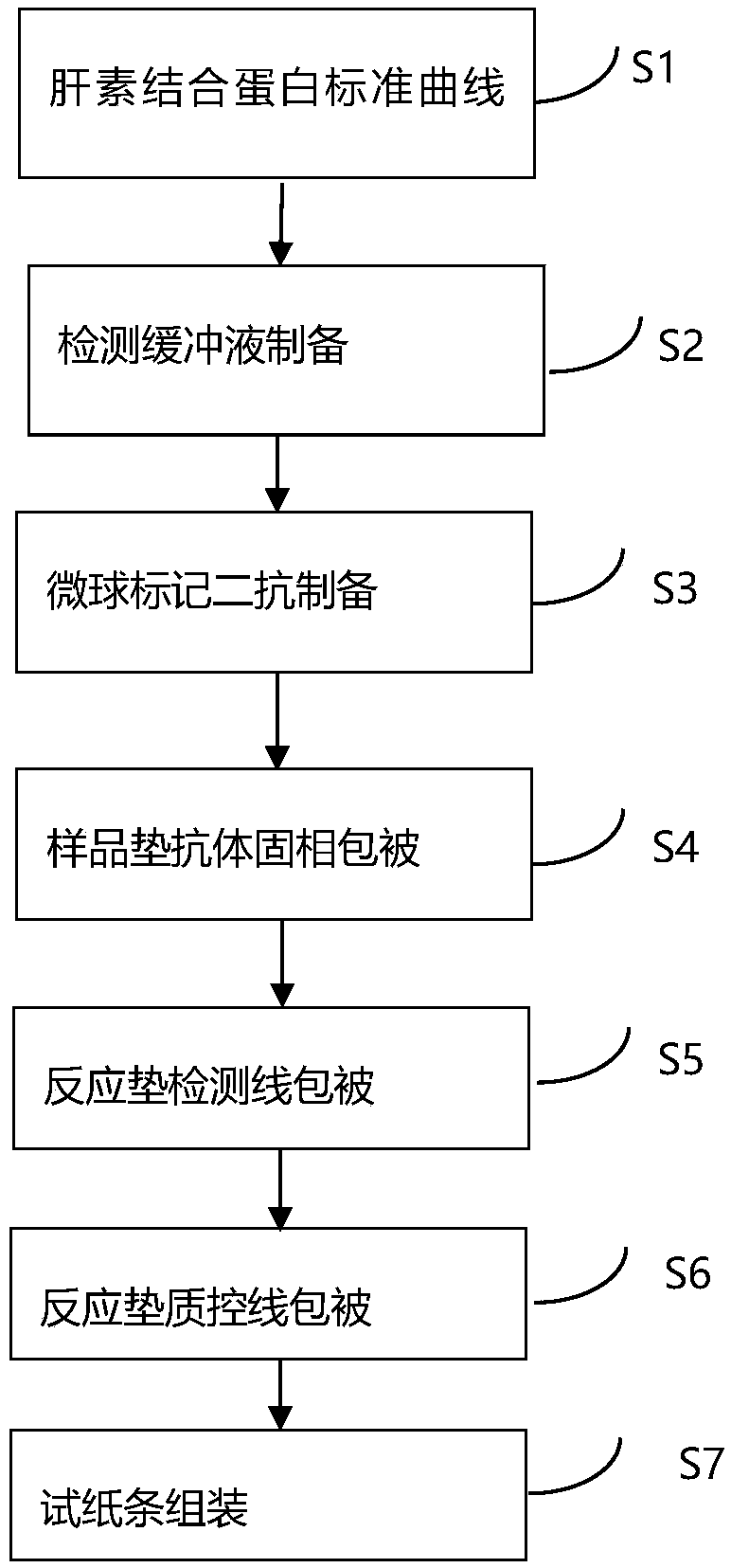
[0039] Such as figure 1 Shown, a kind of immune microsphere chromatography detects the heparin-binding protein detection test paper preparation method, comprises the following steps:
[0040] S1. Specific steps of calibration curve preparation:
[0041] Specific steps: use normal human standard serum (purchased from Jiangsu Enmoase) to prepare 5ug / ml heparin-binding protein mother solution, dilute the mother solution heparin-binding protein standard product, and the heparin-binding protein dilution concentration is 0.1ug / L, 0.5ug / L, 1ug / L, 5ug / L, 10ug / L, 50ug / L, 100ug / L, 150ug / L, 200ug / L, 250ug / L, the signal value and concentration curve were calibrated with heparin-binding protein detection test paper and POCT immunoanalyzer.
Embodiment 2
[0043] S2. Preparation of detection buffer containing heparin-binding protein antibody
[0044] Specific steps: prepare rabbit anti-human heparin binding protein antibody (biocare) with PBS buffer solution of pH 7.4 and 10mM, the preparation concentration is 2ng / ul, add 5% sucrose, 5% BSA, 0.1% Tween-20 , 0.05% sodium azide.
Embodiment 3
[0046] S3. Preparation of microsphere-labeled secondary antibody
[0047] Specific steps: Take 100ml of 10% 0.3um fluorescent microspheres, wash with PH4.5 50mM MES solution for 3 times, add 1ml of freshly prepared 10mg / ml EDAC solution (prepared in PH4.550mM MES), incubate at room temperature for 30min , and then centrifuged at 2000rpm for 5min, then washed with PH4.550mM MES solution, incubated overnight at room temperature with 1ml of 1ug / ul chicken anti-rabbit secondary antibody (PBS), incubated with 10% BSA, 100×Tris-EDTA for 1 hour at room temperature , washed with 1ml PBS three times after centrifugation, stored in 4% sucrose, 0.05% sodium azide solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com