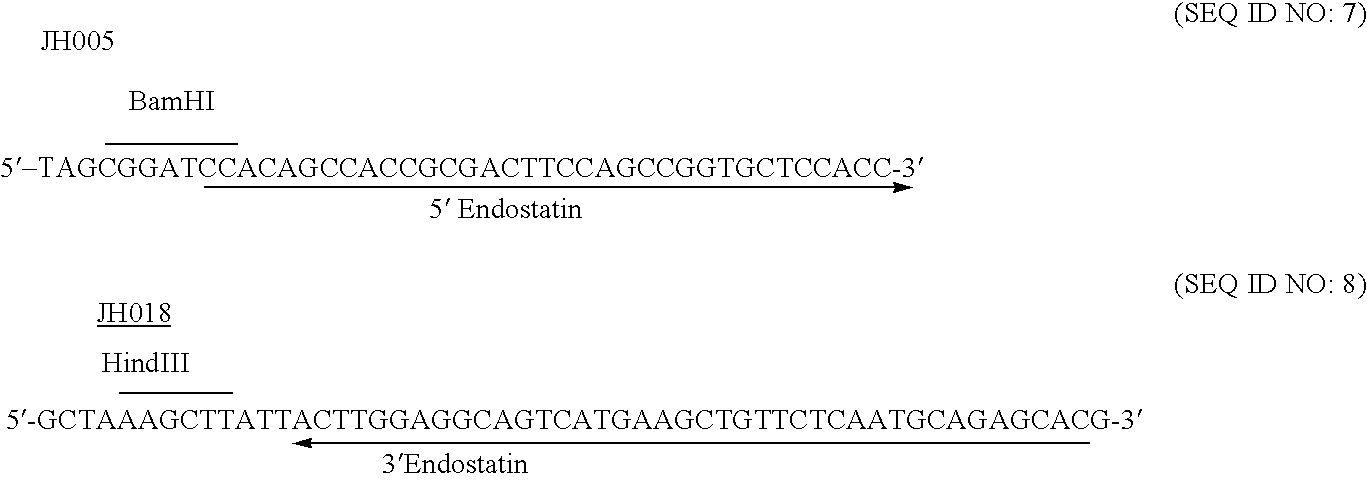Patents
Literature
673 results about "Cell-cell fusion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cell fusion is an important cellular process in which several uninuclear cells (cells with a single nucleus) combine to form a multinuclear cell, known as a syncytium. Cell fusion occurs during differentiation of muscle, bone and trophoblast cells, during embryogenesis, and during morphogenesis.
Fusion partner for production of monoclonal rabbit antibodies
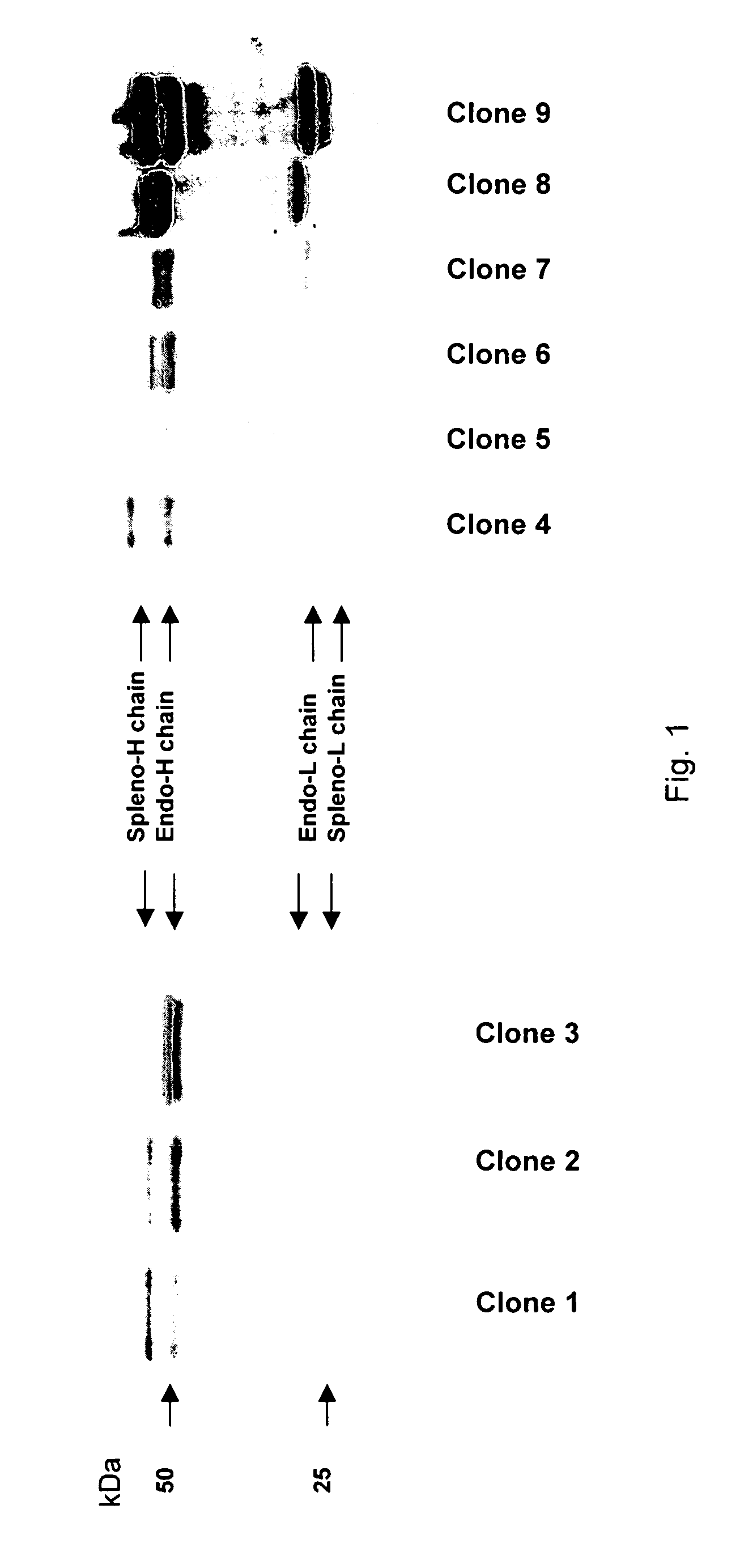
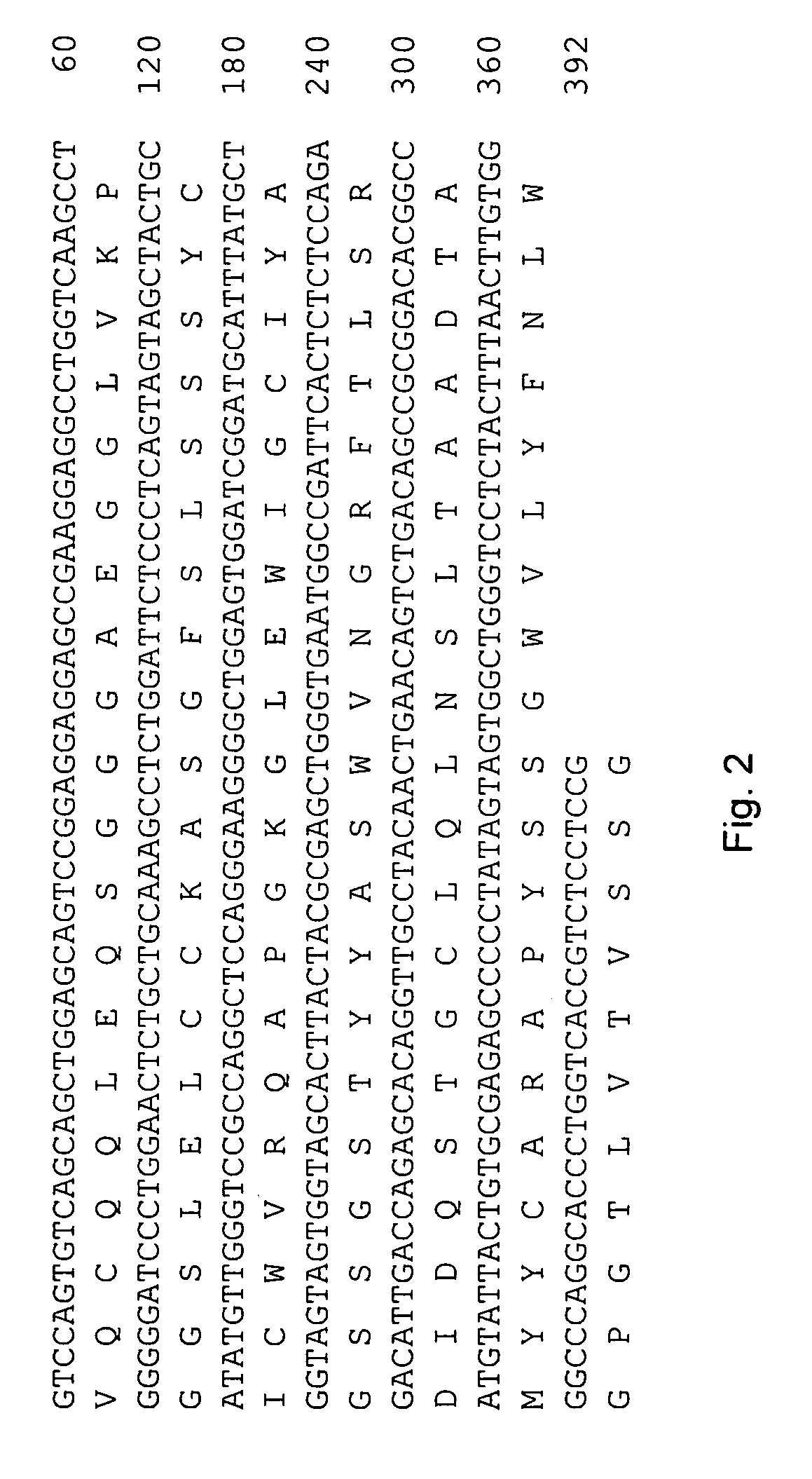
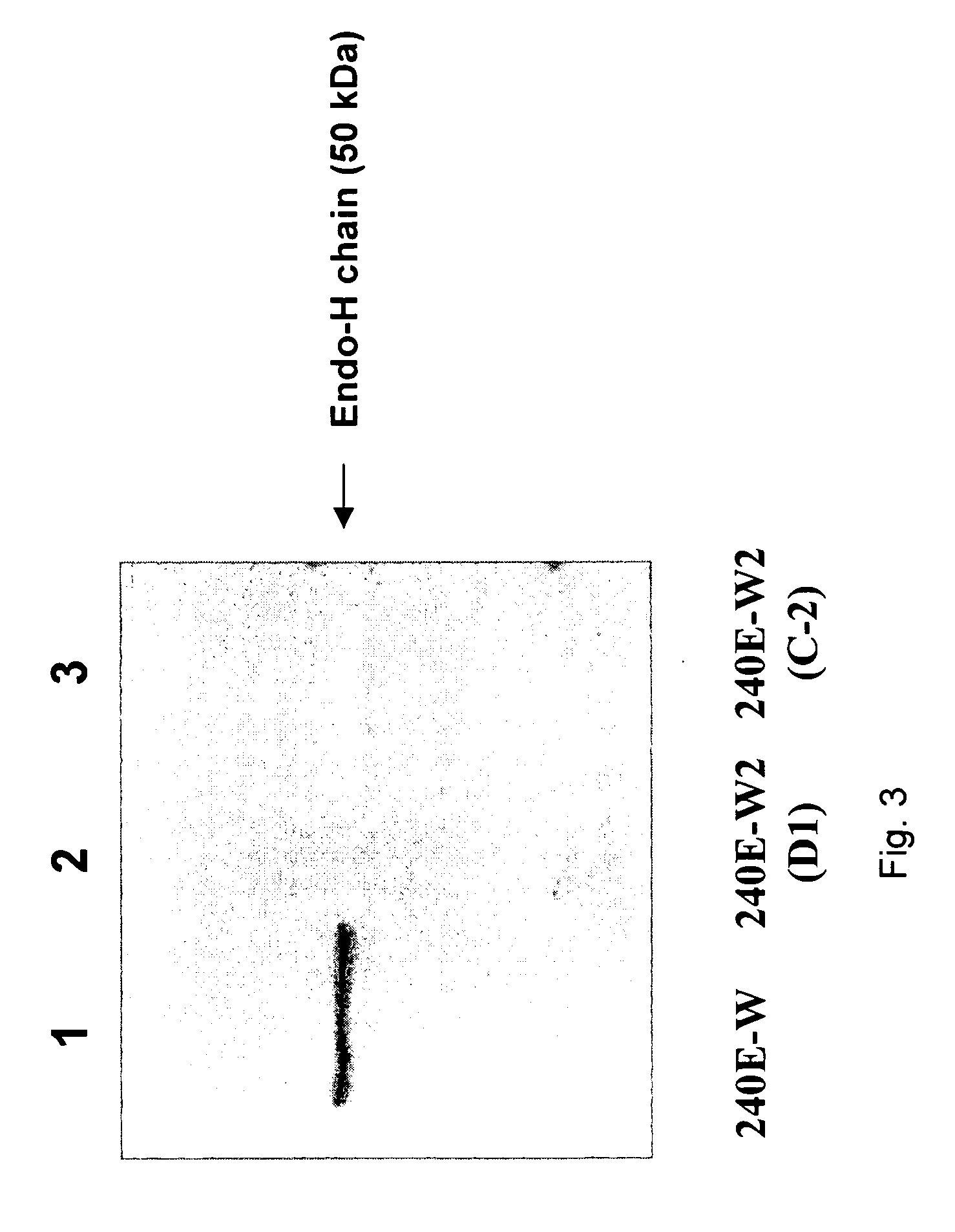
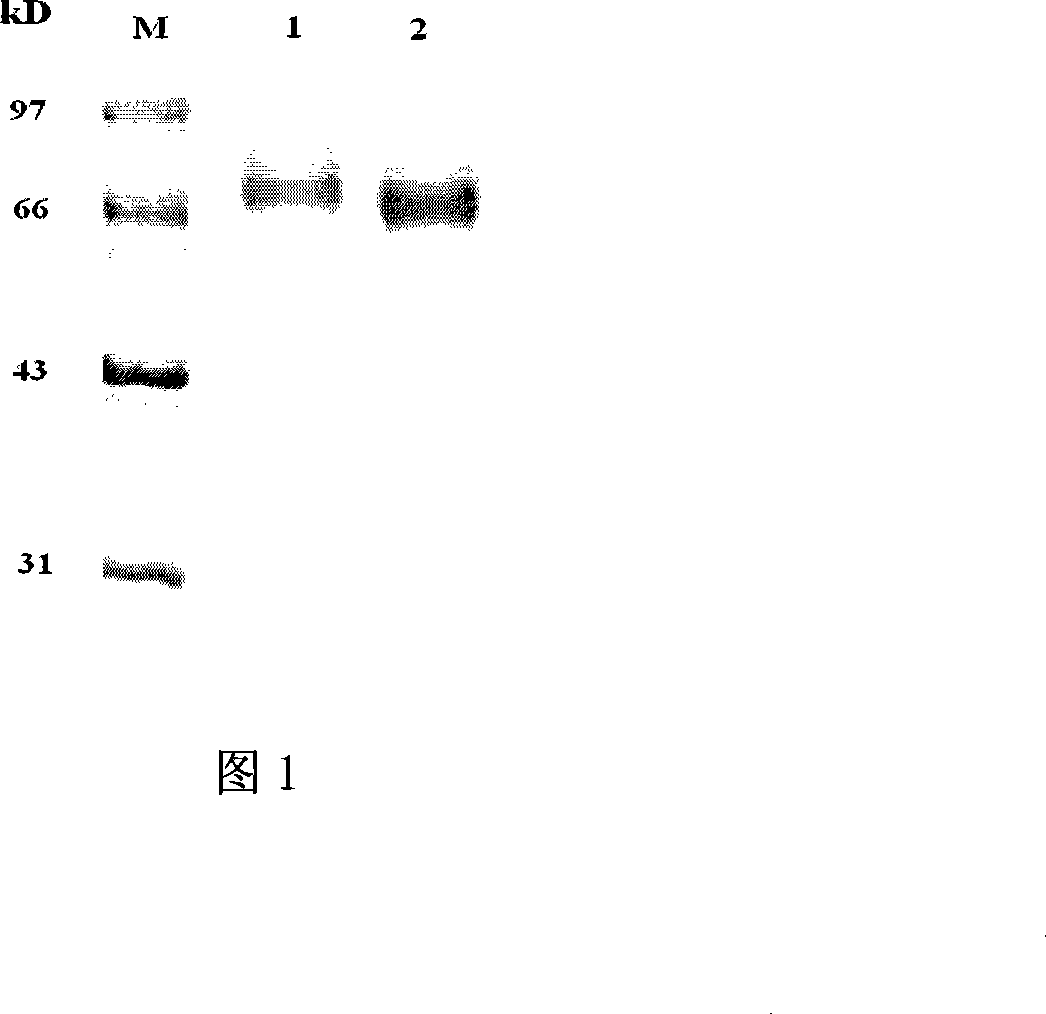
The invention provides a rabbit-derived immortal B-lymphocyte capable of fusion with a rabbit splenocyte to produce a hybrid cell that produces an antibody. The immortal B-lymphocyte does not detectably express endogenous immunoglobulin heavy chain and may contain, in certain embodiments, an altered immunoglobulin heavy chain-encoding gene. A hybridoma resulting from fusion between the subject immortal B-lymphocyte and a rabbit antibody-producing cell is provided, as is a method of using that hybridoma to produce an antibody. The subject invention finds use in a variety of different diagnostic, therapeutic and research applications.
Owner:EPITOMICS INC
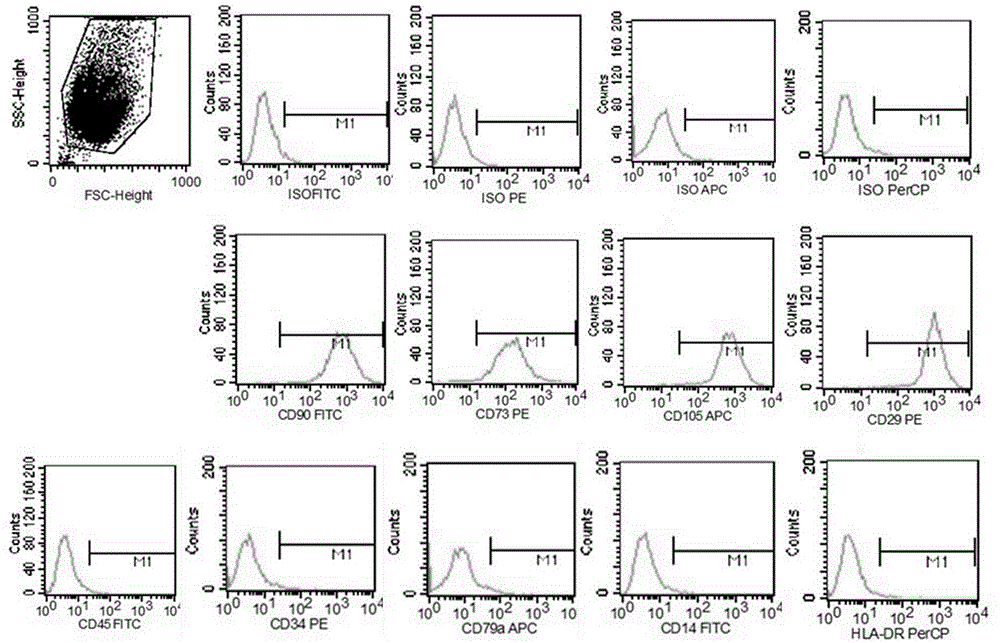
Method for separating mesenchymal stem cells from placenta
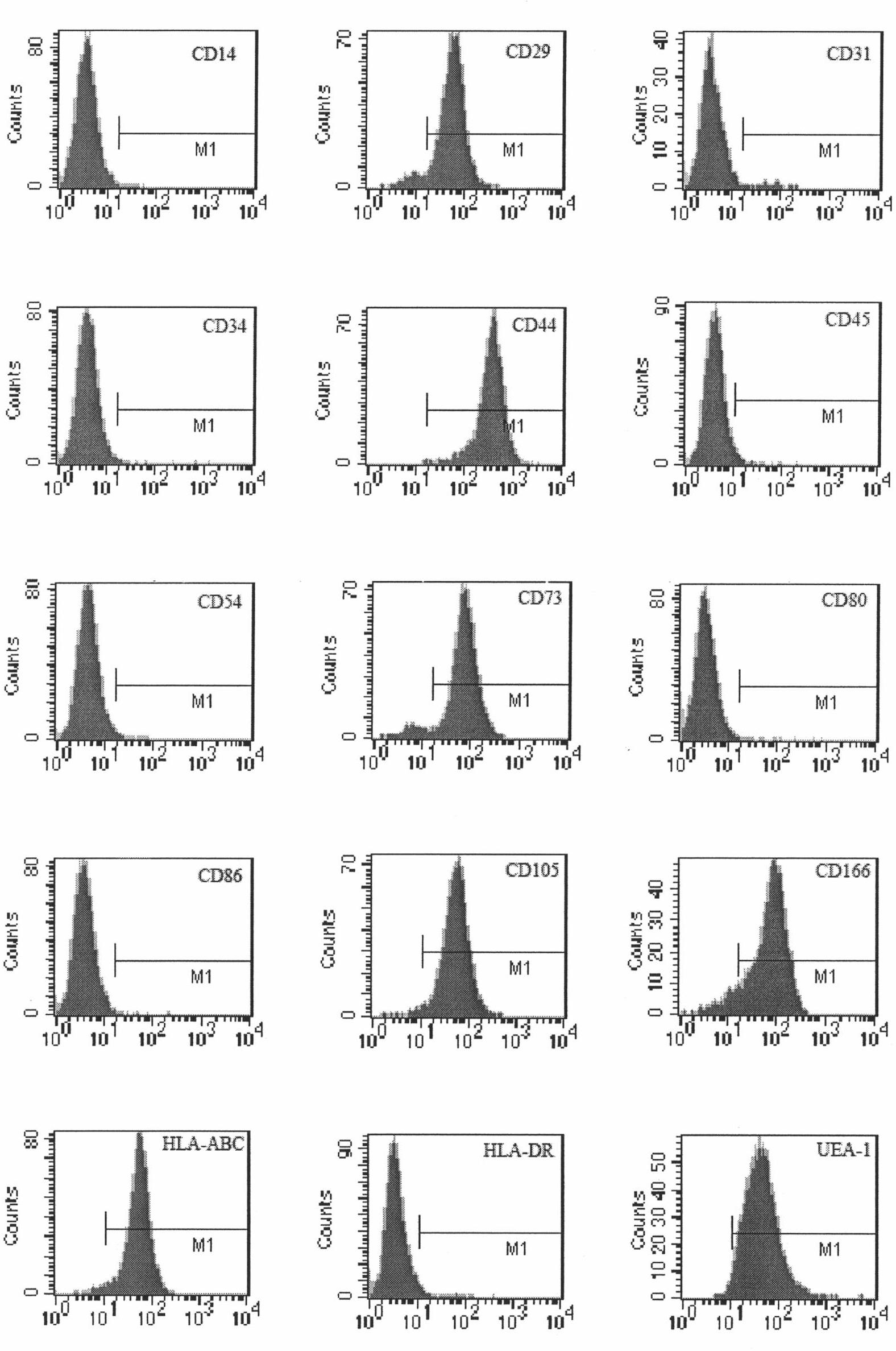
ActiveCN102676451AHigh purityEasy to operateSkeletal/connective tissue cellsEmbryonic cellsFiltrationMesenchymal stem cell
The invention relates to a method for separating mesenchymal stem cells from placenta. The method comprises the following steps: (a) taking placental cotyledon, and fully washing by using a phosphate buffer solution (PBS) to remove residual blood from the placenta; (b) cutting the placental cotyledon into blocks, adding a PBS containing tissue digestive enzyme, and incubating and digesting at 37DEG C; (c) filtering the tissue blocks by using a copper net, and grinding if necessary to promote filtration; (d) centrifuging the collected filtrate, separating mononuclear cells, suspending the obtained cells by using a mesenchymal stem cell (MSC) culture medium, and culturing in a 5 percent CO2 incubator at 37DEG C; and (e) after the dispersed cells form clones, selecting the clone cells, respectively culturing by using an MSC culture medium, and after the cells are fused, performing digestion and passage by using pancreatin to obtain the mesenchymal stem cells of the placenta. By the method, high purity mesenchymal stem cells of the placenta can be obtained.
Owner:BOYALIFE
Method of preventing virus: cell fusion by inhibiting the function of the fusion initiation region in rna viruses having class i membrane fusogenic envelope proteins
ActiveUS20060280754A1Prevent and inhibit infectionSsRNA viruses negative-senseSsRNA viruses positive-senseCell membraneDisease cause
The present invention relates to a method of preventing or inhibiting viral infection of a cell and / or fusion between the envelope of a virus and the membranes of a cell targeted by the virus (thereby preventing delivery of the viral genome into the cell cytoplasm, a step required for. viral infection). The present invention particularly relates to the families of RNA viruses, including the arenaviruses, coronaviruses, filoviruses, orthomyxoviruses, paramyxoviruses, and retroviruses, having Class I membrane fusion proteins as the fusion proteins that mediate this fusion process. The present invention provides for a method of identifying a conserved motif or domain called the fusion initiation region (FIR) in these viruses. The present invention further provides for methods of preventing infection by such viruses, by interfering with their FIR. The present invention further provides for methods of treatment and prophylaxis of diseases induced by such viruses.
Owner:TULANE EDUCATIONAL FUND THE ADMINISTRATORS OF THE
Anti-viral compositions comprising heterocyclic substituted phenyl furans and related compounds
InactiveUS20060287319A1Potent anti-HIV activityInhibit HIV replicationBiocideAntiviralsFuranFluorescence
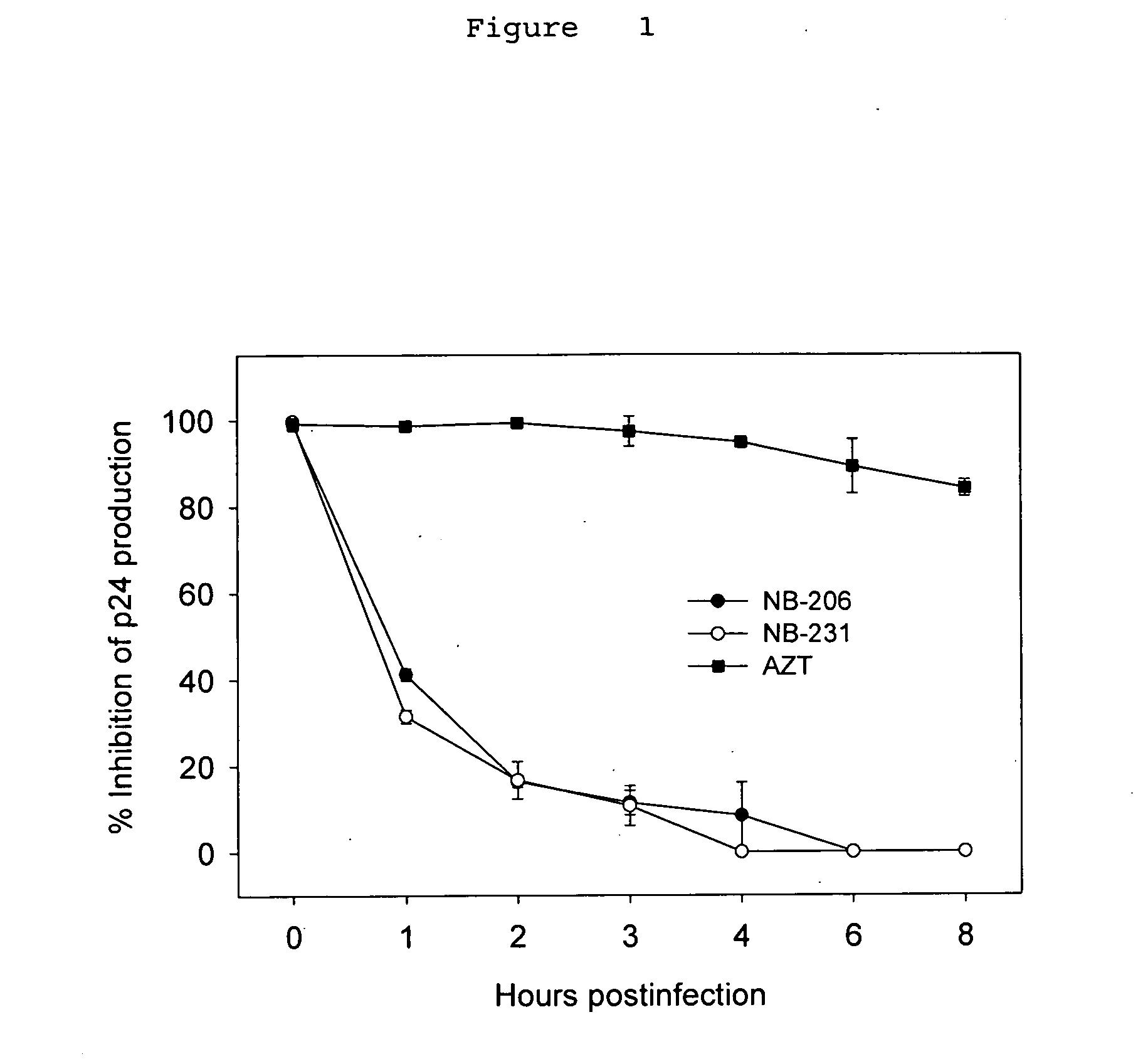
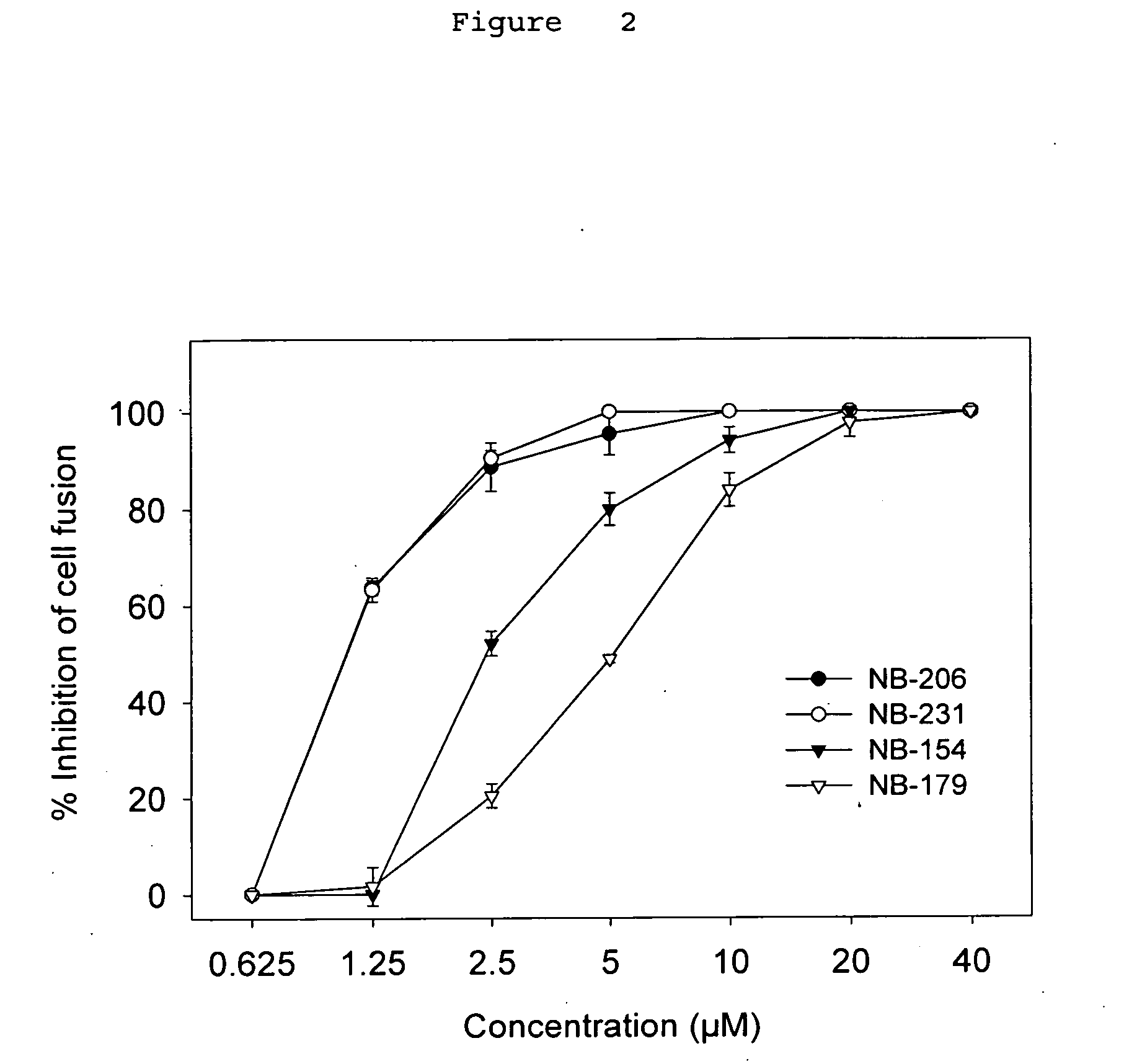
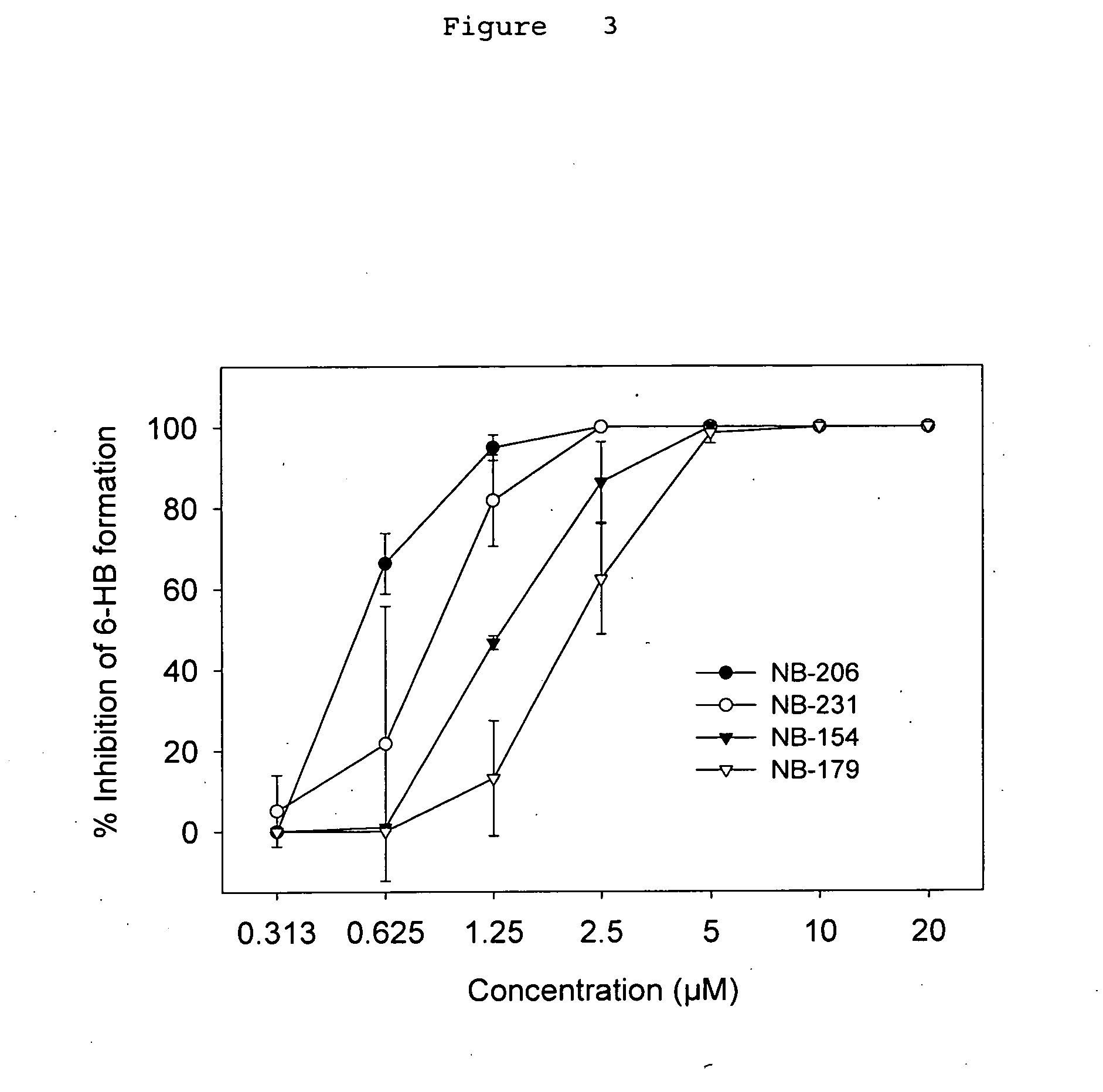
A group of compounds that inhibit HIV replication by blocking HIV entry was identified. One representative compound, designated NB-206, and its analogs inhibited HIV replication (p24 production) with IC50 values at nanomolar levels. It was proved that NB-206 and its analogs are HIV entry inhibitors by targeting the HIV gp41 since: 1) they inhibited HIV-mediated cell fusion; 2) they inhibited HIV replication only when they were added to the cells less than one hour after virus addition; 3) they blocked the formation of the gp41 core that is detected by sandwich enzyme linked immunosorbent assay (ELISA) using a conformation-specific MAb NC-1; and 4) they inhibited the formation of the gp41 six-helix bundle revealed by fluorescence native-polyacrylamide gel electrophoresis (FN-PAGE). These results suggested that NB-206 and its analogs may interact with the hydrophobic cavity and block the formation of the fusion-active gp41 coiled coil domain, resulting in inhibition of HIV-1 mediated membrane fusion and virus entry.
Owner:NEW YORK BLOOD CENT
Hiv inhibiting proteins
InactiveUS20060241027A1High activityProlong half-life in vivoBiocidePeptide/protein ingredientsHiv transmissionAlbumin
The invention relates to proteins comprising HIV fusion inhibiting peptides, such as T-20 and / or T-1249 peptides (including, but not limited to, fragments and variants thereof), which exhibit anti-retroviral activity, fused to albumin (including, but nbot limited to fragments or variants of albumin). These fusion proteins are herein collectively referred to as “albumin fusion proteins of the invention.” These fusion proteins exhibit extended shelf-life and / or extended or therapeutic activity. The invention encompasses therapeutic albumin fusion proteins, compositions, pharmaceutical compositions, formulations and kits. The invention also encompasses nucleic acid molecules encoding the albumin fusion proteins of the invention, as well as vectors containing these nucleic acids, host cells transformed with these nucleic acids and vectors, and methods of making the albumin fusion proteins of the invention using these nucleic acids, vectors, and / or host cells. The invention also relates to compositions and methods for inhibiting HIV-induced cell fusion. The invention further relates to compositions and methods for inhibiting HIV transmission to uninfected cells.
Owner:NOVOZYMES BIOPHARMA DK AS
Separation method and culture method for umbilical cord mesenchymal stem cells
ActiveCN103589683AClear chemical compositionAvoid heterogeneous contaminationSkeletal/connective tissue cellsPenicillinPhosphate
The invention relates to a separation method and a culture method for umbilical cord mesenchymal stem cells. The separation method comprises the following steps: thoroughly cleaning umbilical cord tissue of a healthy newborn by using a PBS (phosphate buffer solution) containing streptomycin and penicillin, and removing blood; shearing the umbilical cord into small sections uniform in length, and mechanically separating, bluntly stripping Wharton' s jelly, and removing umbilical arteries and umbilical veins; uniformly shearing the Wharton' s jelly; re-suspending the sheared Wharton' s jelly through an MSCs (mesenchymal stem cells) culture medium, inoculating to a culture dish with laid gelatin, and putting in a CO2 culture box for cultivation; conducting centrifugal separation to obtain tissue blocks and a cell resuspension solution. The culture method comprises the following steps: enwrapping the culture dish, discarding the gelatin, and washing with the PBS; inoculating the separated out tissue blocks and the cell resuspension into the culture dish; performing digestive subculture after cell fusion growth rate reaches 80-90%.
Owner:BEIJING DONGFANG HUAHUI BIOMEDICAL TECH
Detecting method for aflatoxins
InactiveCN1588068ARealize monitoringEasy to detectComponent separationBiological testingMicroorganismBiological cell
The invention relates to a assaying method for the aflatoxin, specifically saying, it is a method of adopting immunity affinity column and fluorophotometer prepared by monoclonal antibody of aflatoxin to assayt the aflatoxin in the food, which belongs to biocytology and microbacteria toxic metabololic products assaying method technical field.The method is as following: first, preparing monoclonal antibody by the steps involving: immunizing mouse, cell fusion, screening crossing tumour and obtaining the cell line of the crossing tumourc, gathering monoclonal antibody, etc, then using the abovementioned monoclonal antibody to prepare the immunoaffinity column, last, after milling the food and feed, extracting the aflatoxin out of it with methanol / water solution, after filtering, letting the liquid sample flow through the affinity column to be refined, successively, eluting the AFT from the affinity column with methanol, and assaying the content of aflatoxin in the sample with a fluorophotometer.
Owner:SHANGHAI UNIV
Detection for zearalenone
A detection method includes preparing single cloning antibody by steps of antigen synthesis, mouse immune, cell fusion, hybrid tumor selection and its cell line collection, extracting ZEN in corn sample by methanol / aqueous solution; filtering and deluting it; flowing liquid sample through affinity column and it by methanol; and using fluoresclence spectrophotometer to measure out content of zearlenone in set sample solution.
Owner:SHANGHAI UNIV
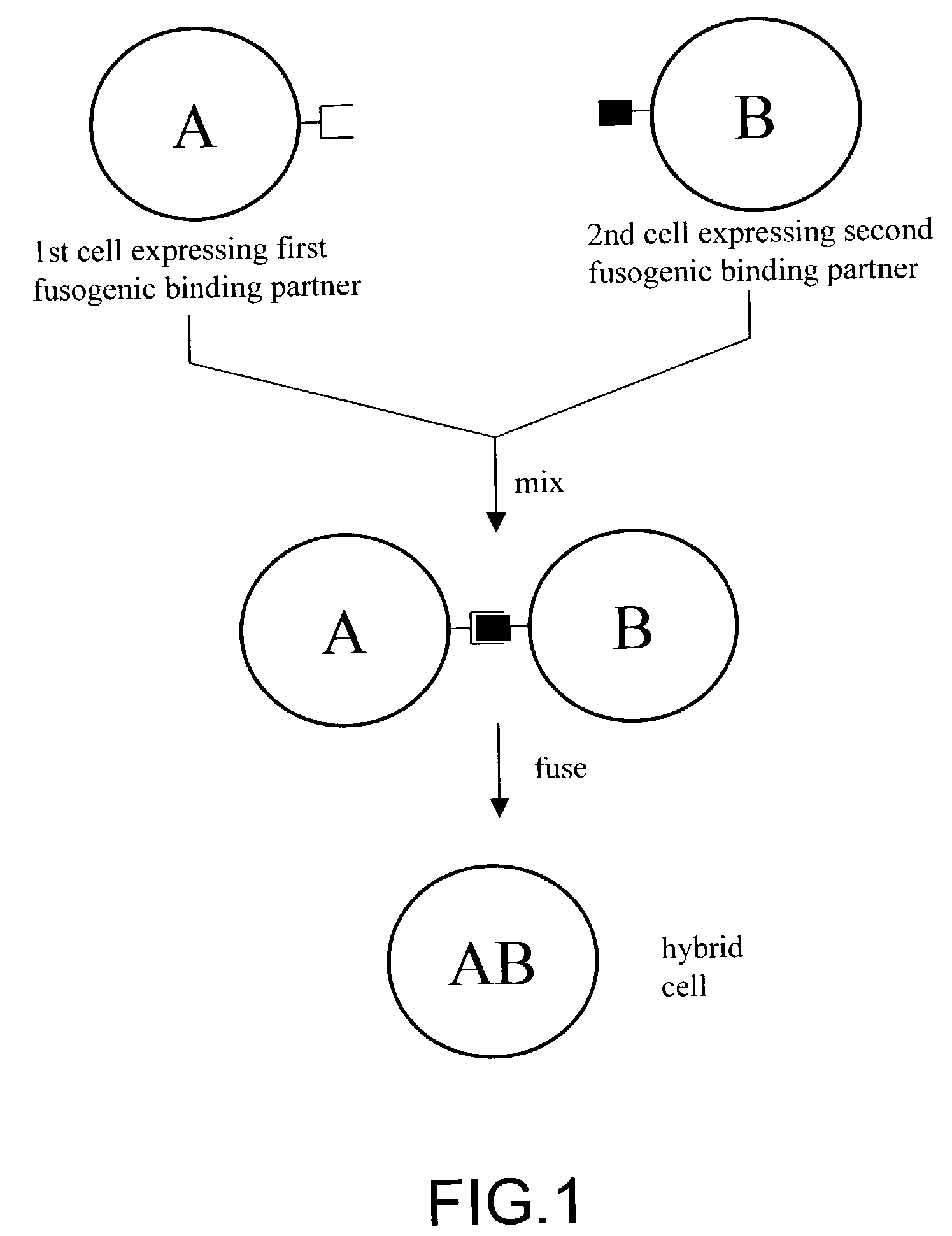
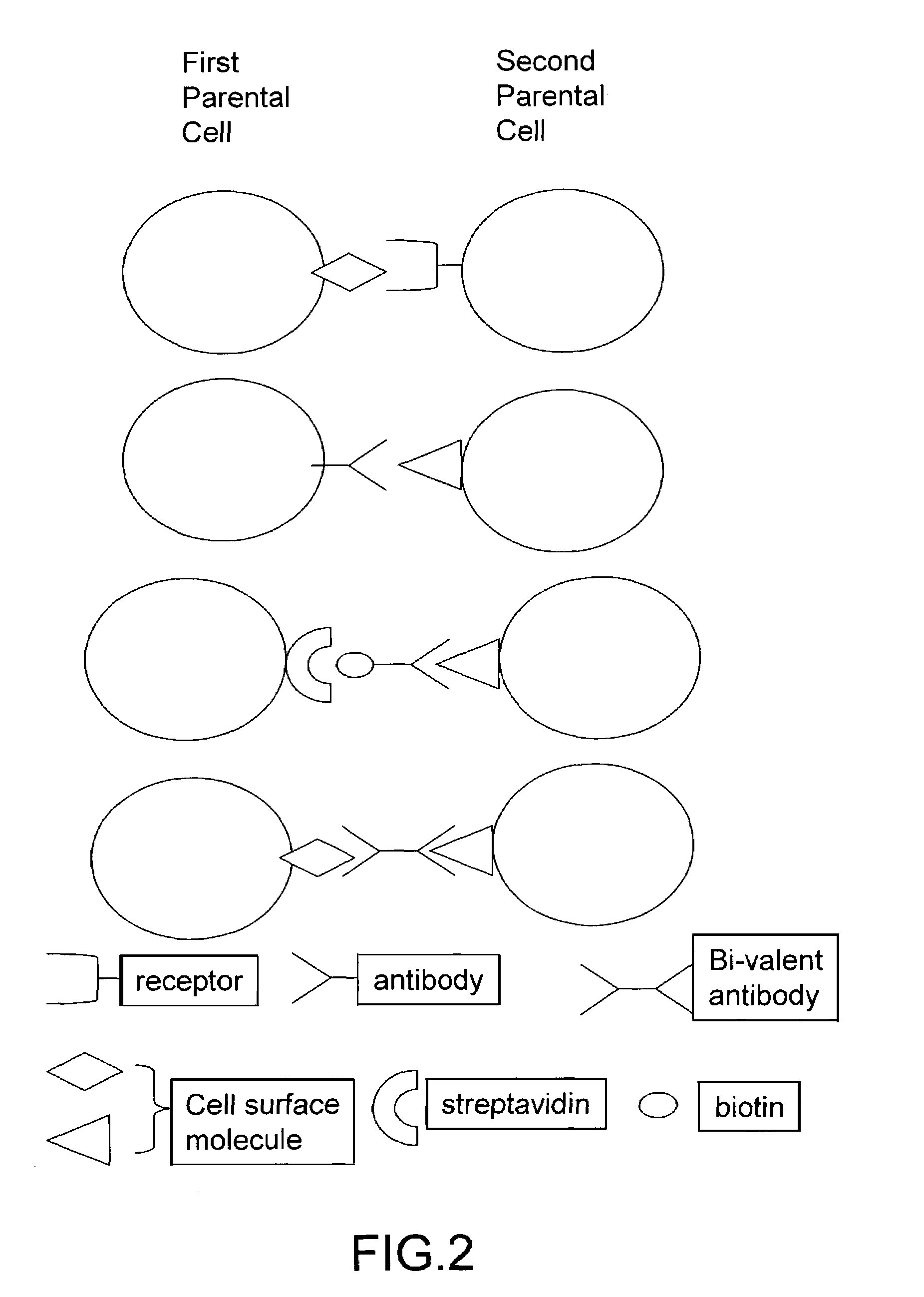
Cell fusion method
ActiveUS7402409B2Increase ratingsAnimal cellsSugar derivativesPolyethylene glycolAntibody-Producing Cells
The invention provides methods for fusing a first cell with a second cell to form a hybrid cell. The methods involve incubating a first parental cell producing a first partner of a fusogenic binding partner pair on its surface with a second parental cell producing a second partner of the fusogenic binding partner pair on its surface. In certain embodiments, the parental cells are incubated with a known fusogen such as polyethylene glycol and the fusogenic binding partner pair increases the rate of cell fusion. In many embodiments, the first cell is an antibody producing cell, the second cell is an immortal cell, and the hybrid cell is a hybridoma cell that produces a monoclonal antibody. Also provided by the invention are methods for producing hybridoma cells, and methods for screening those cells for production of a monoclonal antibody of interest. The invention further provides systems and kits for carrying out the subject methods. The subject methods, systems, and kits find use in a variety of different industrial, medical and research applications.
Owner:EPITOMICS INC
Albumin-fused anti-angiogenesis peptides
InactiveUS20060122374A1High activityExtended half-lifeOrganic active ingredientsFungiAbnormal tissue growthLymphatic Spread
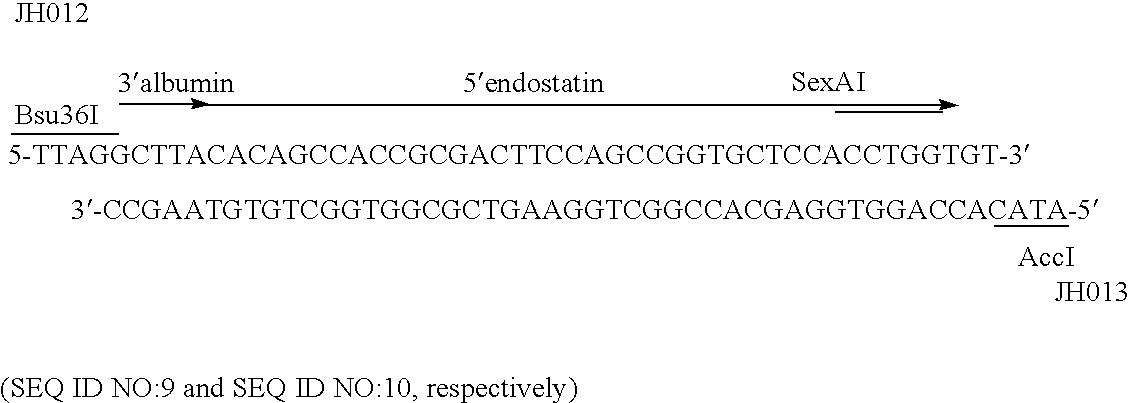
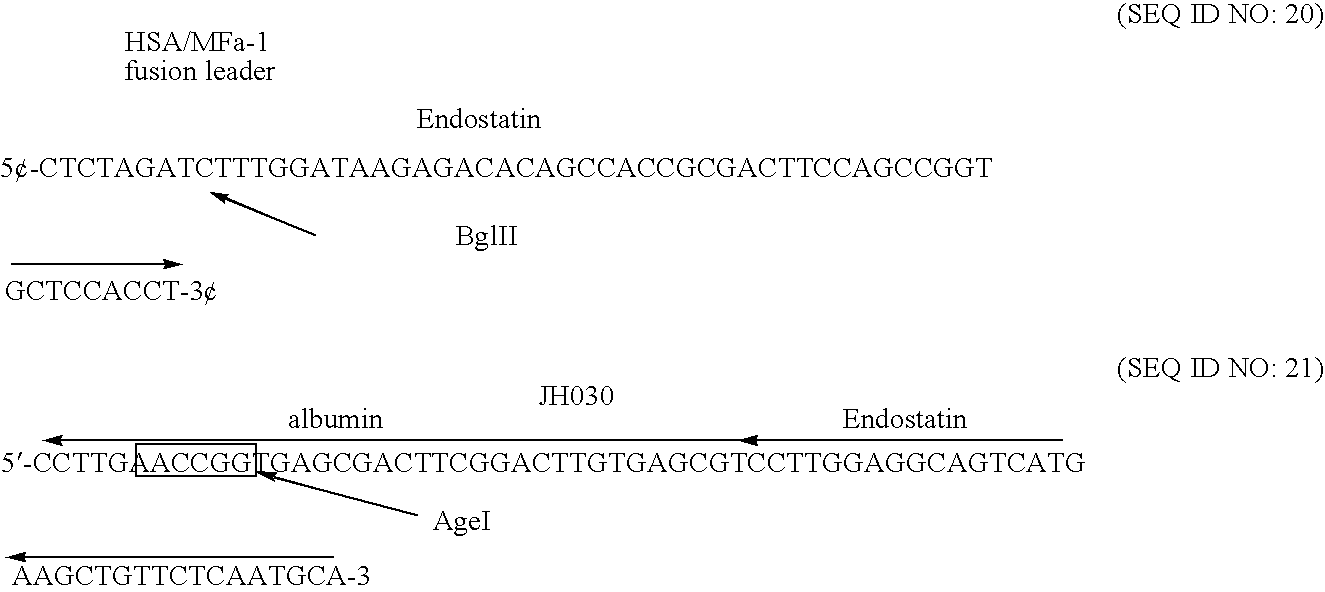
The invention relates to proteins comprising angiogenesis inhibiting peptides, such as endostatin peptides (including, but not limited to, fragments and variants thereof), which exhibit anti-retroviral activity, fused or conjugated to albumin (including, but not limited to fragments or variants of albumin). These fusion proteins are herein collectively referred to as “albumin fusion proteins of the invention.” These fusion proteins are herein collectively referred to as “albumin fusion proteins of the invention.” These fusion proteins exhibit extended shelf-life and / or extended or therapeutic activity in solution. The invention encompasses therapeutic albumin fusion proteins, compositions, pharmaceutical compositions, formulations and kits. The invention also encompasses nucleic acid molecules encoding the albumin fusion proteins of the invention, as well as vectors containing these nucleic acuds, host cells transformed with these nucleic acids and vectors, and methods of making the albumin fusion proteins of the invention using these nucleic acids, vectors, and / or host cells. The invention also relates to compositions and methods for inhibiting proliferation of vascular endothelial cells and tumor aniogenesis induced cell fusion. The invention further relates to compositions and methods preventing growth of, or promoting regression of, primary tumors and metastases; and for treating cancer, diabetic retinophathy, progressive macular degeneration or rheumatoid arthritis.
Owner:NOVOZYMES BIOPHARMA DK AS
Hybridoma cell strain capable of secreting monoclonal antibodies to quinolones and application of monoclonal antibodies thereof
InactiveCN102618502AStrong specificityHigh sensitivityTissue cultureImmunoglobulinsBALB/cAntibody types
The invention discloses a hybridoma cell strain capable of secreting monoclonal antibodies to quinolones and application of the monoclonal antibodies thereof. Ciprofloxacin (CIP) coupled with bovine serum albumin is used as an antigen to immunize BALB / c mice and cell fusion, screening and cloning are carried out so as to obtain one hybridoma cell strain 1F1 capable of stable passage and secretion of monoclonal antibodies (MAb) to quinolones, wherein, the accession number of the hybridoma cell strain 1F1 is CGMCC No. 5608. The titres of ascitic fluids of the 1F1 monoclonal antibodies are up to 10<-7>, and the type and the subclass of the monoclonal antibodies are IgG1 and kappa chain. According to indirect competitive ELISA analysis, the 1F1 monoclonal antibodies perform specific reactions to quinolones like ciprofloxacin, enrofloxacin, ofloxacin, danofloxacin, norfloxacin, enoxacin, marbofloxacin, sarafloxacin and difloxacin. An ELISA method, a kit and test paper for detecting residual of quinolones in food are developed by using the 1F1 monoclonal antibodies.
Owner:ZHEJIANG UNIV
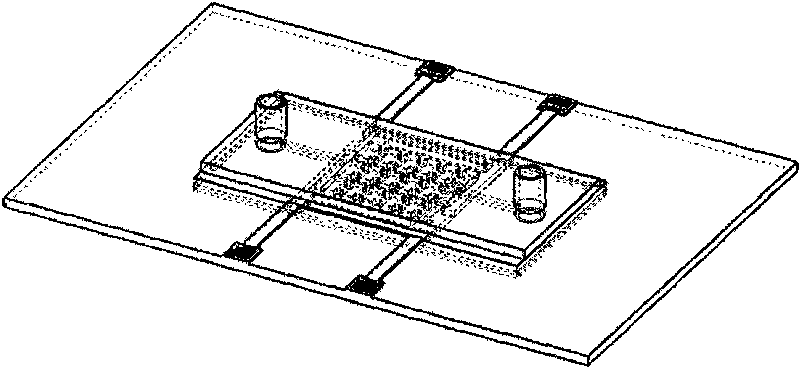
Cell electrofusion chip device based on micro-chamber array structure
InactiveCN101693874AHigh queuing rateImprove fusion rateHybrid cell preparationStress based microorganism growth stimulationElectricityElectrical connection
The invention provides a cell electrofusion microelectrode array chip device based on a micro-chamber structure, which consists of a micro-chamber array chip, a periphery printed circuit board and a flow passage control module. The micro-chamber array chip consists of a quartz substrate layer, a metal microelectrode array, polymer side walls and a polymer water surrounding fender, wherein two opposite metal microelectrodes and two opposite polymer sidewalls form one micro-chamber which is arranged in an array shape; the micro-chamber array chip is bonded on the periphery printed circuit board to form electrical connection; the flow passage control module covers the micro-chamber array chip. In the invention, a certain electrical field is formed in the micro-chamber by outside electrical signals so as to control high-efficient queue and electrofusion of cells inside the micro-chamber, thus realizing that only one pair of cells inside the micro-chamber are subjected to fusion, and improving throughout and safety of cell fusion.
Owner:CHONGQING UNIV
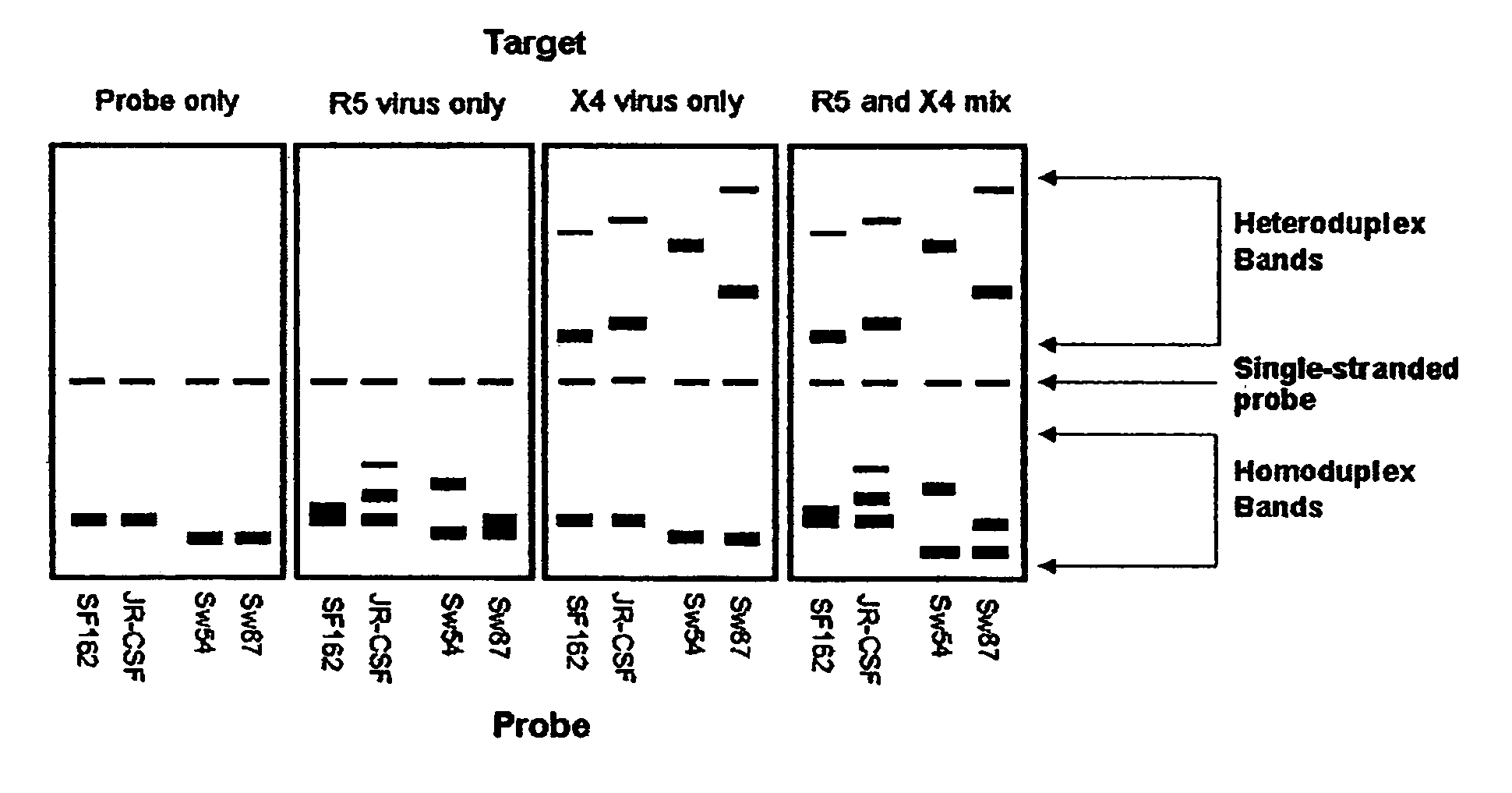
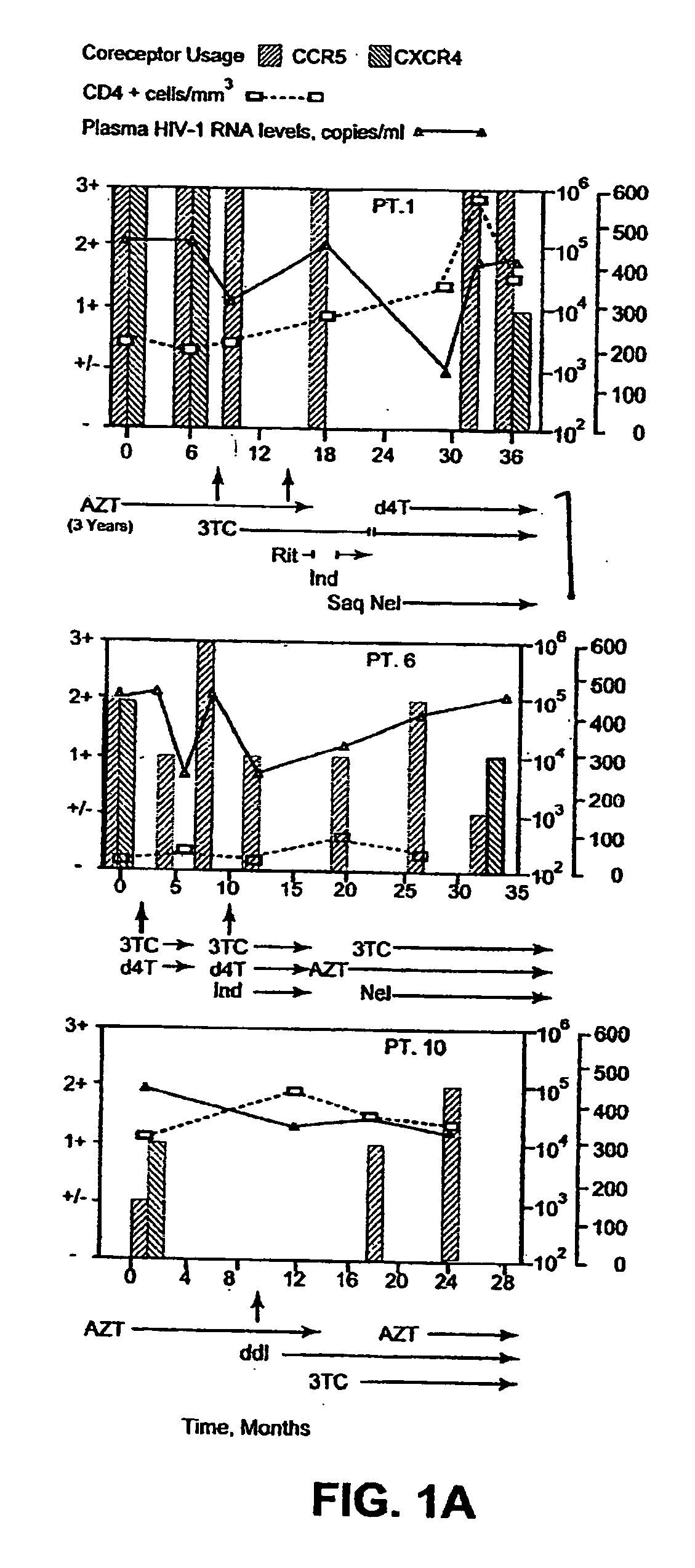
Heteroduplex tracking assay
InactiveUS20060194227A1Accurately predict disease prognosis over timeGood treatment effectMicrobiological testing/measurementVertebrate cellsHeterologousHeteroduplex
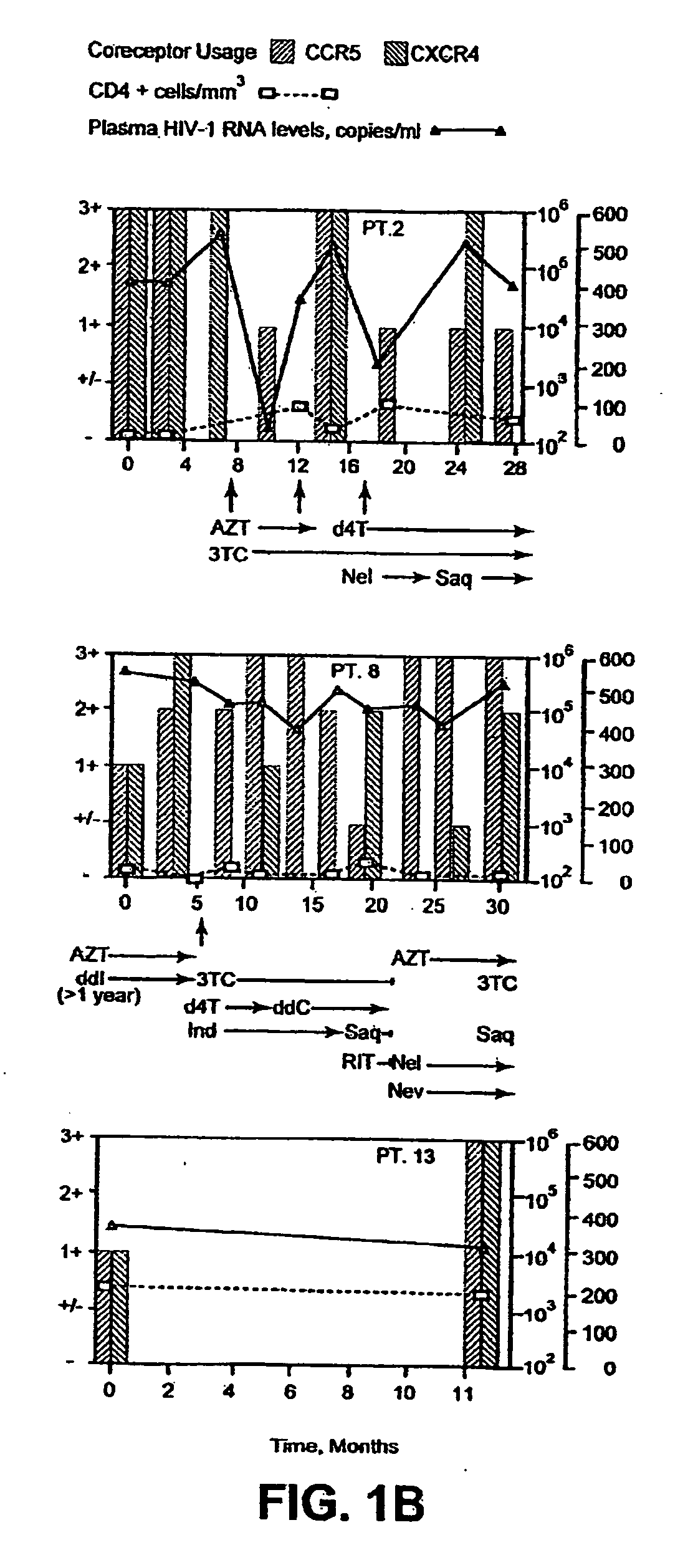
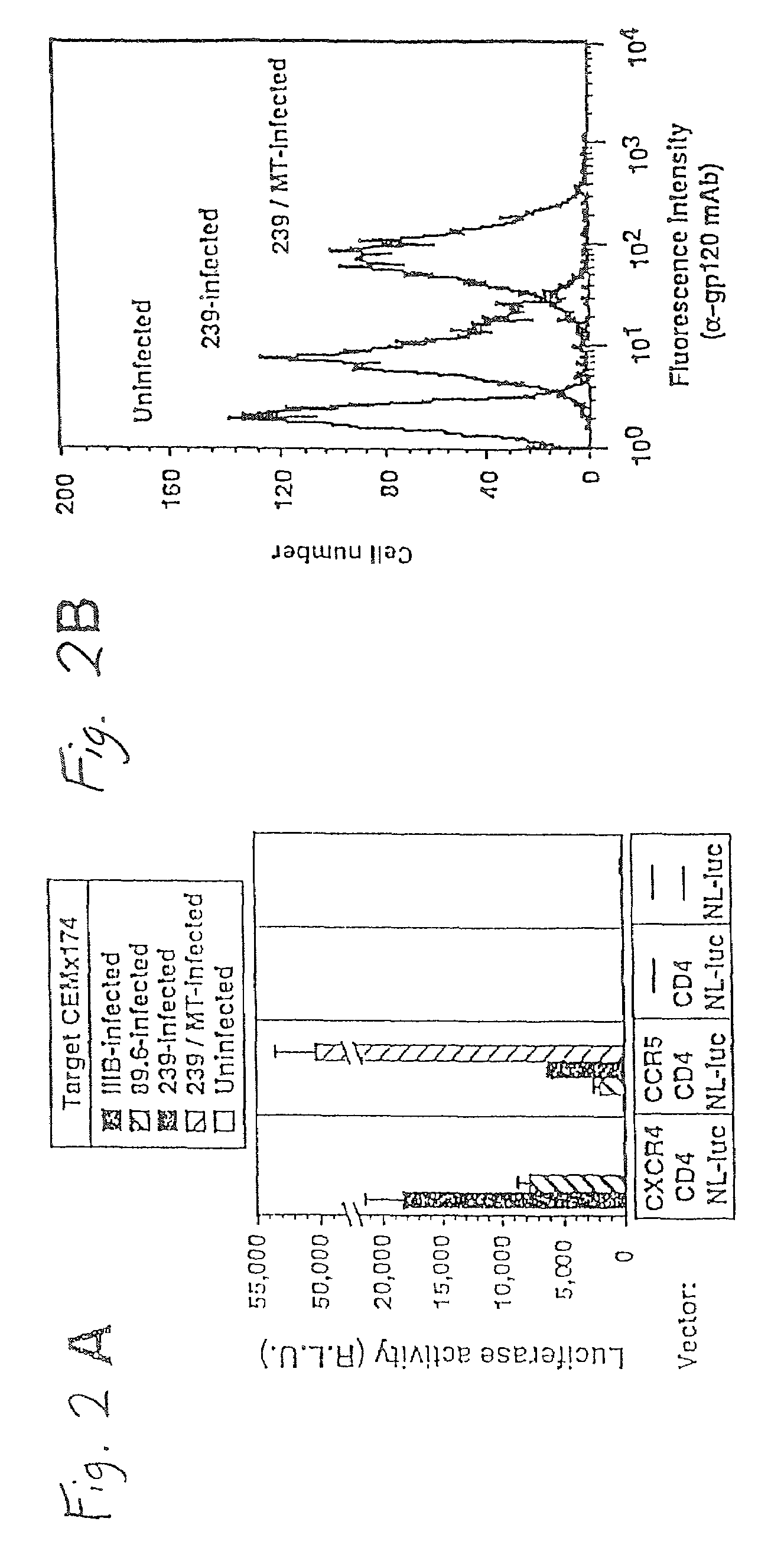
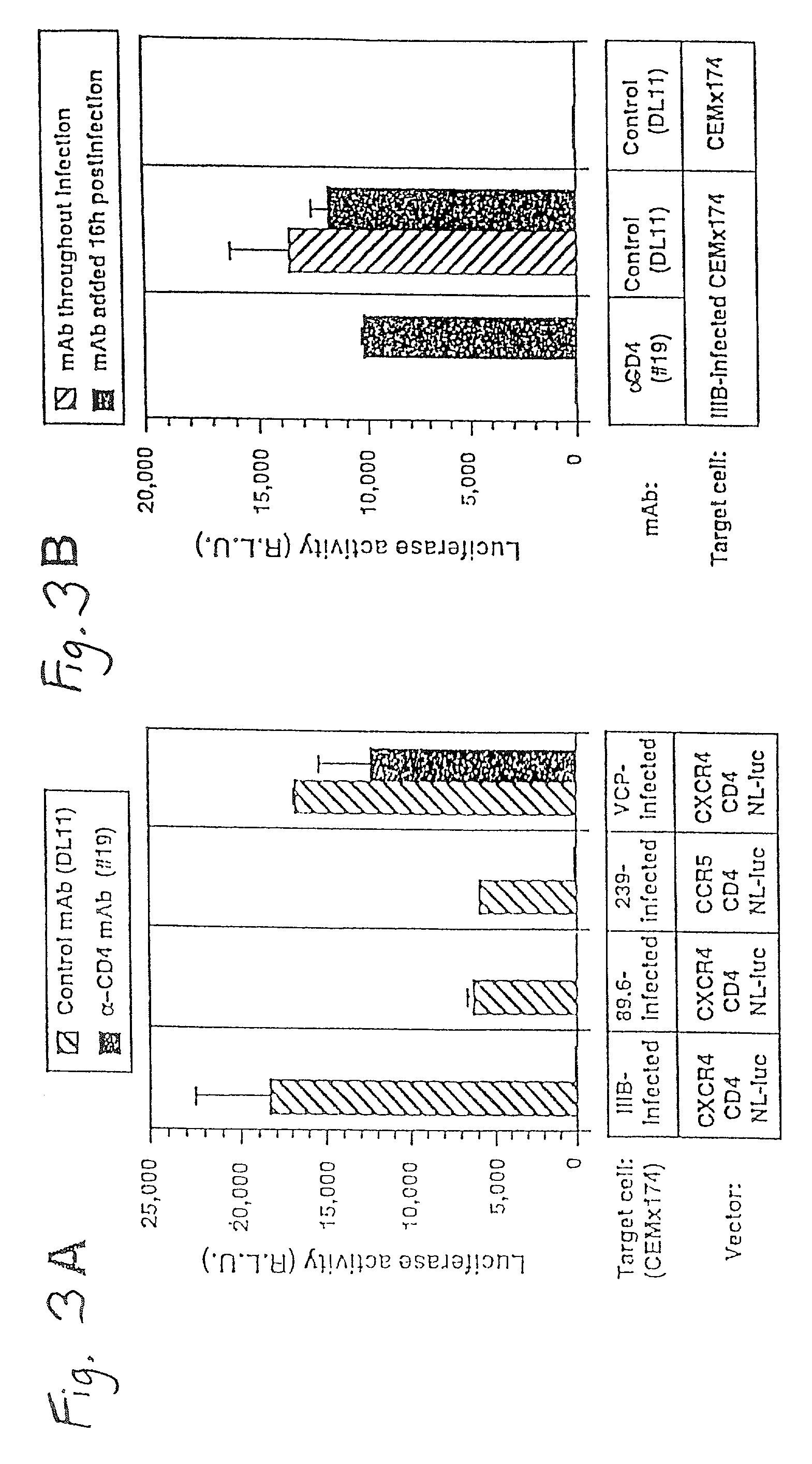
A change in viral tropism occurs in many HIV positive individuals over time and may be indicated by a shift in coreceptor use from CCR5 to CXCR4. The shift in coreceptor use to CXCR4 has been shown to correlate with increased disease progression. In patients undergoing HAART, the predominant populations of virus may be shifted back to CCR5-mediated entry after the CXCR4-specific strains have emerged. The present invention relates to a diagnostic method to monitor coreceptor use in the treatment and clinical management of human immunodeficiency virus (HIV) infection. The present invention further relates to a diagnostic method applied to HIV-positive individuals undergoing HAART to monitor the suppression of CCR5- or CXCR4-specific strains. The diagnostic methods may be used to assist in selecting antiretroviral therapy and to improve predictions of disease prognosis over time. The methods of the invention include cell-based methods, including cell fusion assays, and molecular-based methods, including heteroduplex tracking assay, to both quantitatively and qualitatively analyze patient-derived HIV for coreceptor usage.
Pseudotyping of retroviral vectors, methods for production and use thereof for targeted gene transfer and high throughput screening
ActiveUS20100189690A1Increase in titer and transduction efficiencyHigh transduction efficiencySsRNA viruses negative-senseBiocideSurface markerMorbillivirus
The invention relates to the pseudotyping of retroviral vectors with heterologous envelope proteins derived from the Paramyxoviridae family, genus Morbillivirus, and various uses of the resulting vector particles. The present invention is based on the unexpected and surprising finding that the incorporation of morbillivirus F and H proteins having truncated cytoplasmic tails into lentiviral vector particles, and the complex interaction of these two proteins during cellular fusion, allows for a superior and more effective transduction of cells. Moreover, these pseudotyped vector particles allow the targeted gene transfer into a given cell type of interest by modifying a mutated and truncated H protein with a single-chain antibody or ligand directed against a cell surface marker of the target cell.
Owner:BUNDESREPUBLIK DEUTLAND LETZTVERTRETEN DURCH DEN PRASIDENTEN DES PAUL EHRLICH INSTITUTS
Screening method for hybrid tumor cell monoclonal preparation
ActiveCN101270380ANo training workloadReduce training workloadMicrobiological testing/measurementFermentationScreening methodBiology
The invention relates to a method of realizing the screening and the monoclone preparation of hybridoma cell in one step. The method comprises that the semisolid medium of the monoclone of hybridoma cell is firstly prepared, the basic fibroblast growth factor of human is added into the fusion cell, then hydridoma fusion factor and clone factor are added and well-mixed, then the mixed material and the semisolid medium are gently well-mixed and packaged into plates and cultivated in the condition of 35 DEG C and CO2 with concentration of 5 percent; when visible clone colony grows in the plates, the clone is aspirated from the culture medium by a micropipettor and moved onto a cell culture plate for liquid scale-up culture, and one clone is moved in one hole; till the cell grows to half to two-third of the hole bottom, the culture supernatant is aspirated to do positive detection, and the positive hole is chosen to directly scale-up frozen and stored for further carrying out the characteristic identification of antibody or ascites production. The method of realizing the screening and the monoclone preparation of hybridoma cell in one step has the advantages of the simple operation, the short culture period, not requiring feeder cells and easily achieving the screening and the monoclone preparation of hybridoma cell after cell fusion in one step.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Method for separating and culturing umbilical cord blood mesenchymal stem cells
ActiveCN104630144AIncrease growth rateHigh recovery rateSkeletal/connective tissue cellsPrimary cellBottle
The invention provides a method for separating and culturing umbilical cord blood mesenchymal stem cells. The method comprises the steps of carrying out secondary separation on umbilical cord blood mononuclear cells by using a separating medium, and then inoculating the umbilical cord blood mononuclear cells into a culture bottle in which fibronectin and CD90 monoclonal antibodies are coated; and culturing for 4-5 days by using a serum-free culture system and simulating the in-vivo low-oxygen growth environment of the mesenchymal stem cells, then, removing suspension cells, further culturing adherent cells, and subculturing after the fusion rate of primary cells is up to 60%. By using the method for separating and culturing umbilical cord blood mesenchymal stem cells, provided by the invention, the problems of adherence infirmness, low culture success rate, low purity and the like caused in the extraction and culture processes of the umbilical cord blood mesenchymal stem cells are effectively solved, and the safety in clinical application is improved. In addition, the application ranges of the umbilical cord blood mesenchymal stem cells are widened, the utilization values of the umbilical cord blood mesenchymal stem cells are developed, and the clinical application prospects of the umbilical cord blood mesenchymal stem cells are widened.
Owner:中国医科大学 +1
Clenbuterol complete antigen and method for preparing monoclonal antibody thereof
InactiveCN101182356AHigh sensitivityImprove featuresSerum albuminImmunoglobulins against animals/humansBALB/cAntigen
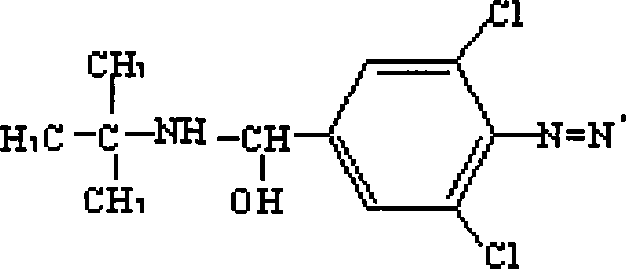
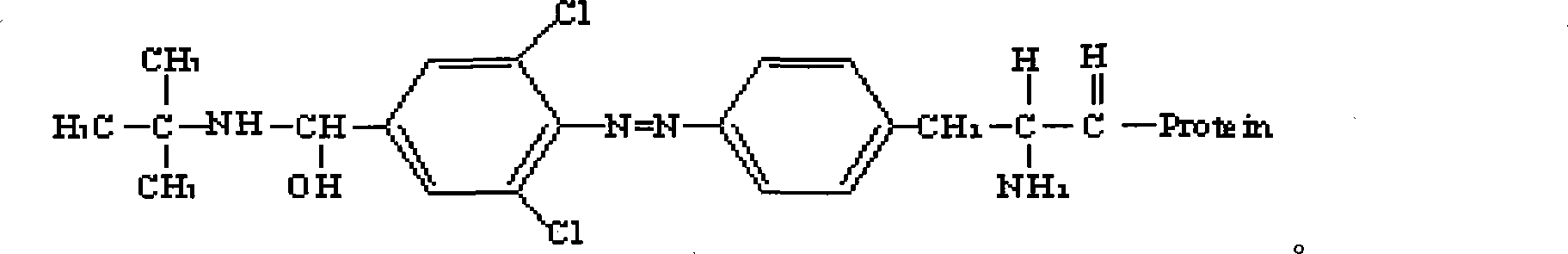
The invention discloses the preparation methods of a complete antigen of clenbuterol hydrochloride and a monoclonal antibody of the clenbuterol hydrochloride. Firstly, the clenbuterol hydrochloride reacts with sodium nitrite under the acid condition to obtain azo clenbuterol hydrochloride; then the azo clenbuterol hydrochloride coupled with bovine serum albumin under the alkaline condition to prepare for the complete antigen of the clenbuterol hydrochloride. A balb / c pure line rat is immunized, after IELISA test shows that the serum of the rat after immunity is eligible, the cell fusion is processed for preparing for the monoclonal antibody of the clenbuterol hydrochloride. The complete antigen of the clenbuterol hydrochloride prepared by the invention can be used for immunizing the animal. The prepared monoclonal antibody can be used for testing the residual quantity of the clenbuterol hydrochloride in meat, meat products, livestock feed and animal body before the animal is slaughtered. The coupling rate of the clenbuterol hydrochloride in the complete antigen of the clenbuterol hydrochloride obtained by the method of the invention with the bovine serum albumin is 17, and a molecular structure formula thereof is as above.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
Method for preparing CysC-paired monoclonal antibody
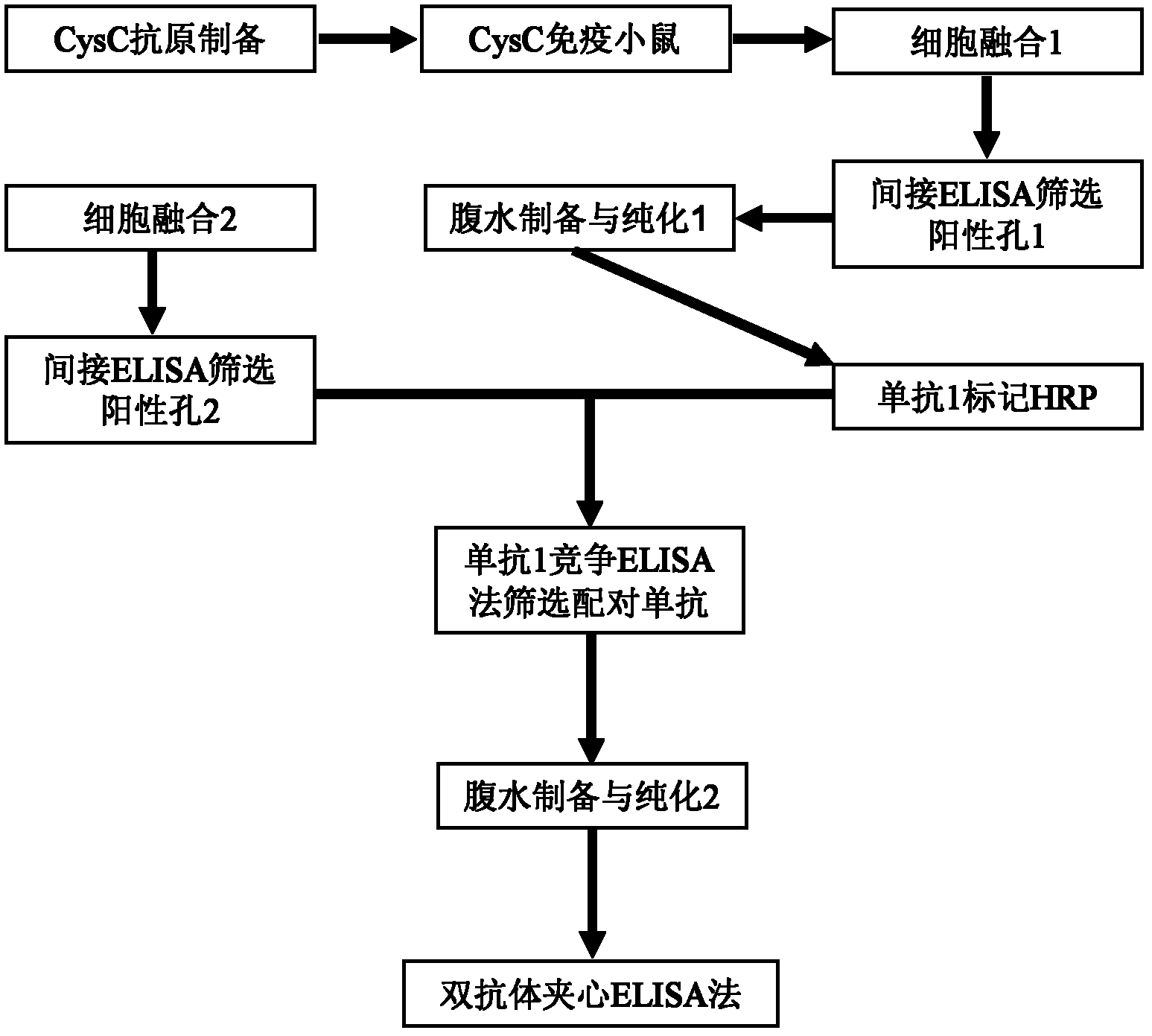
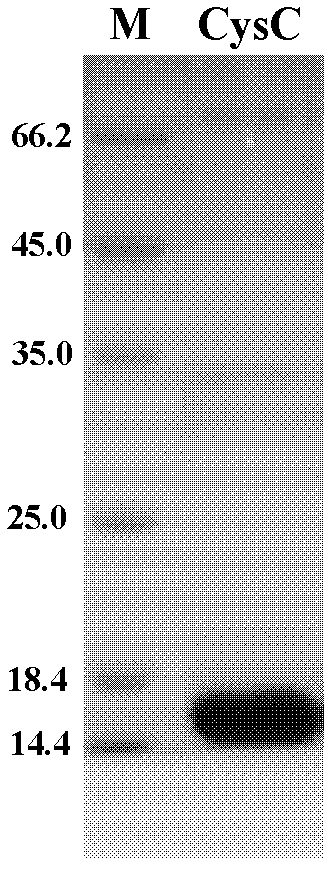
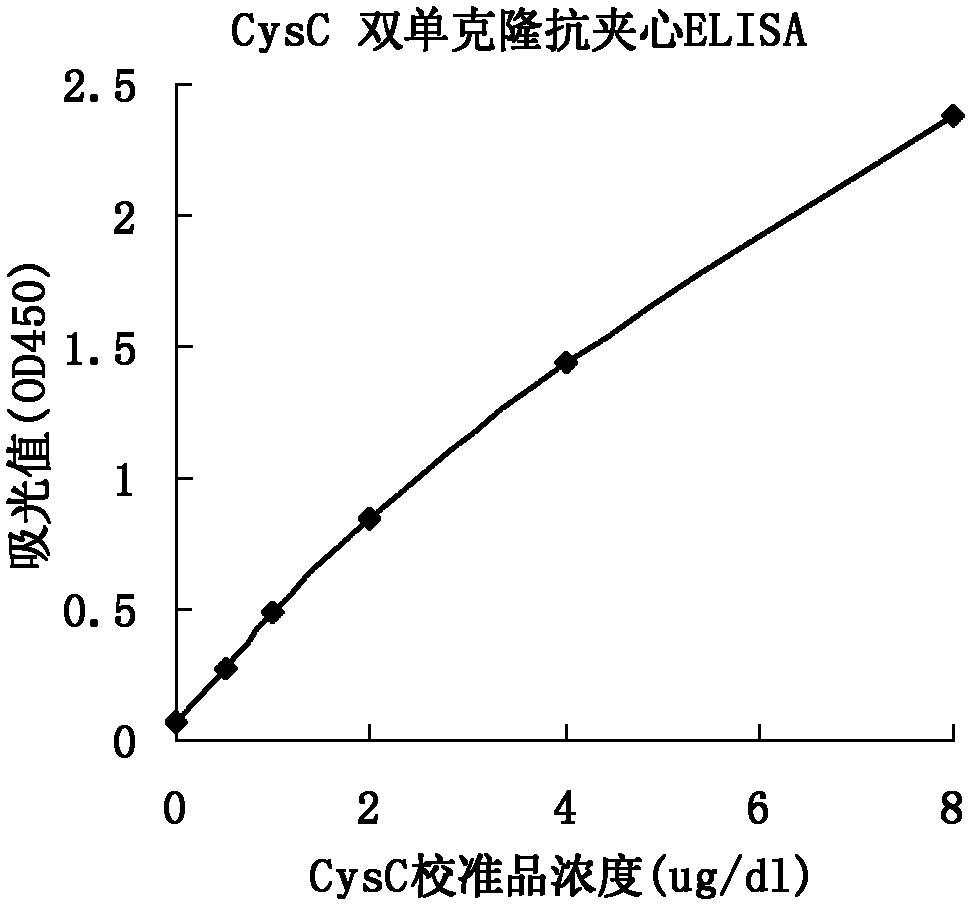
ActiveCN102321176AReduce workloadExpand the scope of the filterImmunoglobulins against protease inhibitorsAntigenPeroxidase
The invention relates to the field of protein engineering, in particular to a method for preparing CysC-paired monoclonal antibodies, which comprises the following steps of: screening hybridoma cells having specific binding with CysC after fusing mouse splenic cells and myeloma cells capable of generating CysC antibodies, preparing cells for generating the monoclonal antibodies, finishing first-time cell fusion, further purifying out the monoclonal antibodies, and selecting one monoclonal antibody to be marked with horse radish peroxidase; then implementing the second-time fusion of the mouse splenic cells and the myeloma cells; finishing CysC-antigen coating on an elisa plate, adding a hybridoma-cell cultural supernatant obtained through the second-time cell fusion to elisa-plate holes, arranging a blank control hole, subsequently adding the monoclonal antibody marked with the horse radish peroxidase to the elisa-plate holes, incubating at the temperature of 37 DEG C for 30 minutes, adding a substrate to each hole for color rendering, and measuring a light absorption value under 450nm after stopping reaction through sulfuric acid; and selecting the uninhibited holes as the paired monoclonal antibodies.
Owner:BEIJING LEADMAN BIOCHEM
Sorghum mosaic virus tri-anti sandwich enzyme-linked immuno sorbent assay kit
InactiveCN101852807AHigh detection sensitivityStrong specificitySerum immunoglobulinsImmunoglobulins against virusesElisa kitPositive control
The invention discloses a sorghum mosaic virus tri-anti sandwich enzyme-linked immuno sorbent assay (TAS-ELISA) kit which comprises positive control, negative control, a sorghum mosaic virus polyclonal antibody, a sorghum mosaic virus monoclonal antibody, an enzyme labeled antibody and other materials and drugs, wherein the sorghum mosaic virus polyclonal antibody is obtained through rabbit immune separation serum after separating and purifying a sorghum mosaic virus sugarcane separator; and the sorghum mosaic virus monoclonal antibody is obtained by mouse immune, cell fusion and clone purification. The TAS-ELISA kit prepared by the invention has high detection sensitivity, strong specificity, high repeatability and high economic usefulness.
Owner:云南省农业科学院生物技术与种质资源研究所
Low-voltage direct-current controlled continuous flow cell electrofusion chip
InactiveCN102174387AImprove pollution preventionImprove the degree of anti-interferenceHybrid cell preparationStress based microorganism growth stimulationLow voltageContinuous flow
The invention provides a microelectrode array chip which can be used for continuous flow cell electrofusion. The chip consists of three layers, namely a substrate, a cover plate and a metal supporting piece, wherein the substrate is provided with a microchannel for cell suspension to continuously flow and a microelectrode array for electrically stimulating cells; and the microelectrode array is engraved on the two sides of the microchannel, is connected with the metal supporting piece by a metal lead through a micropore, and is connected with a direct-current power supply through a wire to lead in an electrical signal. The microelectrode array chip has a simple structure and is provided with a small number of microelectrodes, so that processing difficulty is far lower than that of a high-density microelectrode cell electrofusion chip; the required fusion voltage is direct-current low voltage, the requirement on equipment is low, and device cost is far lower than that of the conventional cell electrofusion method; meanwhile, cell fusion is continuously performed in the process that cell pairs flow through the microchannel, fusion yield is also far higher than that of the conventional cell electrofusion method, so that the popularization and application of the microelectrode array chip are facilitated.
Owner:CHONGQING UNIV
Monoclone antibody of swine fever virus resistant wild strain E2 protein, preparation method and application thereof
InactiveCN101294147ANeutralizing activityImmunoglobulins against virusesTissue cultureSwine Fever VirusCholera
The invention discloses a monoclonal antibody against virulent strain E2 protein of classical swine fever virus and a hybridoma cell strain secreting the monoclonal antibody. The hybridoma cell strain is obtained by using hog cholera lapinized virus vaccine strain E2 protein expressed by Baculovirus as tolerogen, selecting Shimen strain E2 protein as immunogen, immunizing mouse by cyclophosphamide immunosuppression method, carrying out cell fusion, and sieving hybridoma cell strain capable of stably secreting monoclonal antibody against E2 protein. The monoclonal antibody can react with Shimen strain and can produce specific reaction with virulent strain of classical swine fever viruses of 1.1, 2.1, 2.2 and 2.3 gene sub-groups. The monoclonal antibody has neutralization activity and does not react with hog cholera lapinized virus vaccine strain, so that the monoclonal antibody can be used for differentiating virulent strain of classical swine fever virus and hog cholera lapinized virus vaccine strain, which establishes the foundation for establishing a method for differentiating wild virus infection of classical swine fever and vaccine immunity and for researching the molecular difference between CSFV virulent strain and mild strain.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Kit used for rapidly detecting Escherichia coli O157:H7 in sample, and detection method thereof
InactiveCN102435745ASuitable for mass inspectionAccurate detectionMaterial analysisBALB/cEscherichia coli
The invention relates to a kit used for rapidly detecting Escherichia coli O157:H7 in a sample, and a detection method thereof. The invention belongs to the technical field of immunological detection. According to the invention, a heated and deactivated Escherichia coli O157:H7 immunogen is used for immunizing a healthy New Zealand rabbit, such that a polyclonal antibody is obtained, and the polyclonal antibody is adopted as a coating antibody; a BALB / C mouse is immunized, and cell fusion is carried out, such that a monoclonal antibody is obtained, and the monoclonal antibody is adopted as a secondary antibody; and a double-antibody sandwich ELISA kit of Escherichia coli O157:H7 in foodstuffs (meat) is established. With the kit, a rapid and highly efficient detection means is provided for the detection of the residue of Escherichia coli O157:H7 in foodstuffs. The kit is advantaged in relatively low cost, relatively good stability, and relatively good repeatability. According to the invention, a detection limit is 105cfu / mL. The kit and the method are suitable for large-batch detections of samples.
Owner:王利兵 +2
Virus-like particles (VLPs) comprising heterologous multiple membrane spanning proteins
Enveloped virus vectors are described which comprise a cellular virus receptor protein and which are capable of fusing with a cell which comprises a viral envelope protein to which the cellular virus receptor protein is cognate. Enveloped virus vectors comprising a plurality of cellular virus receptor proteins are also described. Methods for making the enveloped virus vectors are described, as are methods of using the enveloped virus vectors. The invention further relates to a lipoparticle comprising a membrane spanning protein, and the lipoparticle can be attached to a sensor surface. The invention relates to methods of producing and using the lipoparticle to, inter alia, assess protein binding interactions.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Anti-rabbit hemorrhagic disease virus VP60 albumen monoclonal antibody
InactiveCN101519447AThe preparation method is simple and feasibleStrong specificityImmunoglobulins against virusesTissue cultureBALB/cCell culture supernatant
The invention relates to an anti-rabbit hemorrhagic disease virus (RHDV)VP60 albumen monoclonal antibody, and belongs to the technical field of biology. An SP2 / 0 myeloma cell and a BALB / c mouse splenic cell immunized by utilizing RHDV to recombine VP60 albumen undergo cell fusion, are selectively cultured by an HAT culture medium and undergo double ELISA screening by utilizing the recombined VP60 albumen and RHDV; the obtained cell culture supernatant is checked up and screened respectively to obtain a hybrid tumor cell strain A3C which can stably excrete the anti-RHDV VP60 albumen monoclonal antibody; the ascitic fluid ELISA titer of the A3C is detected to be 1:327,600; and according to identification, the monoclonal antibody can specifically combine the expressed recombined VP60 albumen as well as the RHDV, and one single reaction strip appears in both specific combinations, thereby proving that the anti-RHDV VP60 albumen monoclonal antibody is a VP60 specific antibody of the RHDV capsid albumen.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Cell membrane tumor vaccine and preparation method and application thereof
InactiveCN110090298AImprove immunityGood biocompatibilityFused cellsCancer antigen ingredientsAbnormal tissue growthDendritic cell
The invention discloses a cell membrane tumor vaccine and a preparation method and an application thereof. For the first time, exogenous tumor antigens and adoptive cells are not used, a cell fusion technology is used to fuse dendritic cells and tumor cells, and after culture, the whole tumor antigen and costimulatory molecules are expressed on the cell membrane of the fused cells. A nano cell membrane vaccine prepared by coating the nanoparticle with the cell membrane subjected to peeling of the fused cell can directly activate the T cell like the antigen presenting cell, can also be recognized by the dendritic cell, processed and presented to the T cell, and indirectly activates the T cell. Two T cells are combined for activation of pathways, which can cause the strong anti-tumor immuneresponse. A cell membrane tumor vaccine strategy that mimics the tumor cells and the antigen presenting cells enables the development of the vaccines against a variety of tumor types, and has good biocompatibility and system safety. Therefore, the cell membrane vaccine is very suitable for the field of tumor vaccines.
Owner:WUHAN UNIV
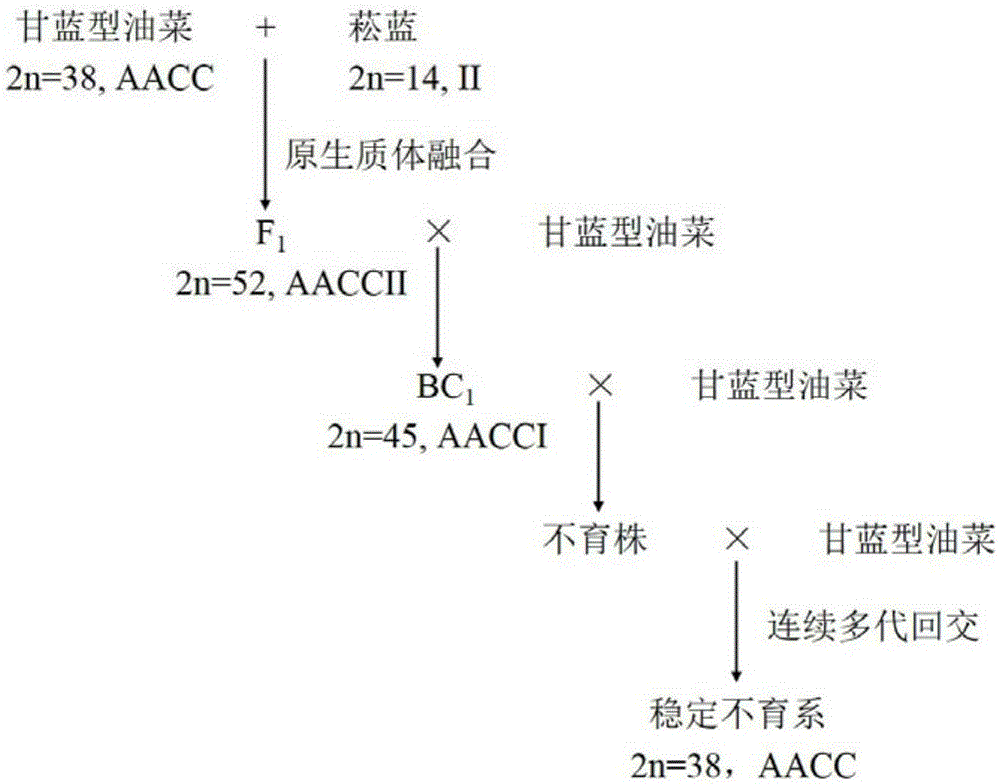
Breeding method of cabbage type rape woad oil cytoplasm male sterile line
InactiveCN105230486AStable sterility traitsExpansion of sterile cytoplasmic resourcesPlant tissue cultureHorticulture methodsProtoplastSomatic cell
The invention belongs to the technical field of rape molecular breeding, and specifically relates to a breeding method of cabbage type rape woad oil cytoplasm male sterile line. The invention also relates to a protoplast fusion technology to recombinate the cytoplasm and nuclear genome of cabbage type rape-woad so as to establish cytoplasmic male sterility of recombinant cytoplasm. The breeding process comprises the following steps: obtaining the leaf meat protoplast of cabbage type rape-woad through separation and extraction, obtaining the protoplast fused somatic cell hybrid of cabbage type rape-woad through a cell fusion method; taking the obtained somatic cell hybrid of cabbage type rape-woad and leaf meat protoplast cell hybrid of cabbage type rape-woad as the female parent, taking cabbage type rape as the male parent, carrying out multi-generation backcross, and obtaining cytoplasmic male sterile line with carpelloid stamen; wherein the mitochondrion gene PCR amplification verifies that mitochondrial genome carries out recombination during the fusion process, thus the nucleoplasm becomes uneven, and the sterile character appears.
Owner:HUAZHONG AGRI UNIV
Method for cultivating triploid dendrobium huoshanense by crossbreeding method of tetraploid plants and diploid plants
ActiveCN104396722AIncrease productionHigh in polysaccharidesPlant genotype modificationBULK ACTIVE INGREDIENTProtoplast
The invention discloses a method for cultivating triploid dendrobium huoshanense by a crossbreeding method of tetraploid plants and diploid plants. The method comprises the following steps: (1) dendrobium huoshanense species which are from different producing areas and have characteristics of good stress resistance, rapid growth, thick stalk, long stem, many tillers, low fiber content and high active ingredient content are preferably selected as polyploidy cultivated original plant parents; (2) seeds of the different species of original plants undergo aseptic sowing in a solid medium so as to obtain diploid protocorms; (3) protoplasts of the different species of diploid protocorms are prepared and cell fusion is carried out; (4) detected tetraploid plants undergo asexual tissue culture so as to obtain tetraploid plant seedlings; (5) the tetraploid plant seedlings undergo hardening-seedling acclimatization and cultivation to flowering; (6) the tetraploid plants and diploid plants undergo artificial pollination and crossbreeding, and fructification is completed to obtain triploid dendrobium huoshanense seeds; and (7) the triploid seeds undergo aseptic sowing and culture so as to obtain a lot of triploid seedlings.
Owner:WEST ANHUI UNIV
Hybridoma cell line producing monoclonal antibody against foot-and-mouth disease virus, the monoclonal antibody therefrom, immunoassay reagent and kit, and immunoassay method
ActiveUS20110014639A1Useful for developmentImprove efficacyAnimal cellsMicrobiological testing/measurementMonoclonal antibody 14G2AStructural protein
Provided herein are a hybridoma cell line producing monoclonal antibody against foot-and-mouth disease virus (FMDV), the monoclonal antibody therefrom, reagent and kit for ELISA, and immunoassay method. The hybridoma cell line is produced by cell fusion of a parental cell and a myeloma cell line and has the same characteristics as the cell line whose strain designation is CmA40 and deposition number is ATCC (To be Provided). The parental cell is a splenocyte isolated from the spleen of a mouse immunized by an antigen derived from a 3ABC non-structural protein (NSP) of FMDV. The antigen used here is expressed by a prokaryotic cell. The monoclonal antibody produced by the hybridoma cell line can specifically recognize a 3ABC polypeptide and does not cross-react with an antiserum of swine vesicular disease virus.
Owner:NAT INST FOR ANIMAL HEALTH COUNCIL AGRI EXECUTIVE YUAN
Preparation method of deciduous tooth mesenchymal stem cells and used kit
InactiveCN105907711AReduce the risk of infectionEasy to useCulture processDead animal preservationSingle cell suspensionMesenchymal stem cell
The invention discloses a preparation method of deciduous tooth mesenchymal stem cells, comprising the steps of: cleaning and sterilizing the tissue surfaces of deciduous teeth with normal saline and 75% alcohol, preserving the deciduous teeth in deciduous teeth preserving fluid; acquiring dental pulp from the deciduous teeth, adding dental pulp digestive fluid to the dental pulp for digestion; obtaining a unicell suspension; adding cell culture fluid, carrying out centrifuging and cleaning, adding precipitated cells into cell culture fluid, putting the cell culture fluid in a carbon dioxide incubator for culturing; when the primary cell fusion reaches 70%, adding cell washing fluid, shaking the culture bottle for washing cells, sucking and abandoning the cell washing fluid, adding cell digestive fluid for digestion, adding cell culture fluid to terminate digestion, repeatedly beating upon the bottle bottom until the cells completely fall off, adding cell culture fluid, inoculating into a culture bottle, putting the culture bottle in the carbon dioxide incubator for culturing, regarding the cells as P1 generation mesenchymal stem cells; when the P1 generation cell fusion reaches 70-80%, carrying out trypsin digestion, collecting digestive cells, carrying out centrifuging and inoculation; after 3d, carrying out trypsin digestion again, collecting digestive cells, carrying out centrifuging, counting and inoculation, and when the P3 generation cell growth reaches 80%, gathering and cryopreserving the cells.
Owner:ANHUI NEW LIFE STEM CELL TECH CO LTD
Humanized monoclonal antibody targeting Claudin18.2 as well as preparation method and application thereof
ActiveCN112940124AStrong specificityThe preparation method is simple and easyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseHybridoma technology
The invention relates to a humanized monoclonal antibody targeting Claudin18.2 as well as a preparation method and application thereof. According to the present invention, the Claudin18.2 humanized monoclonal antibody obtained through the cell fusion and the hybridoma technology can be highly specifically combined with CHO-Claudin18.2 cells, but is almost not combined with CHO-Claudin18.1 cells, the preparation method of the Claudin18.2 humanized monoclonal antibody of the present invention has simple steps, and the obtained Claudin18.2 humanized monoclonal antibody has good ADCC and CDC activity on the expression of Claudin18.2 positive tumor cells. The humanized monoclonal antibody of Claudin18.2 disclosed by the invention is high in specificity and small in side effect, and has a very good prospect in application to treatment and / or prevention or diagnosis of Claudin18.2-related diseases such as tumors.
Owner:NANJING KAEDI BIOTHERAPEUTICS LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com