Cell membrane tumor vaccine and preparation method and application thereof
A technology of tumor vaccines and cell membranes, applied in the field of biological tumor vaccines, can solve the problems of difficult preservation of immune cells, risk of carcinogenesis, and long time consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
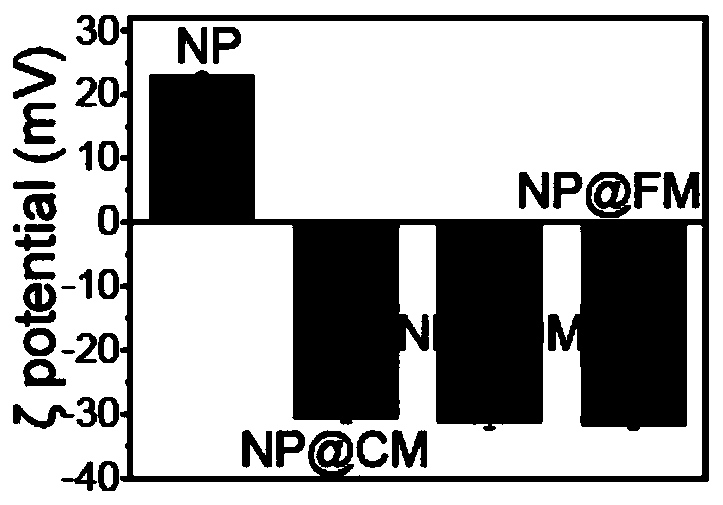
Image
Examples
Embodiment 1
[0053] [Example 1] Preparation of Nano Cell Membrane Tumor Vaccine (NP@FMs)
[0054] 1. Inactivation of tumor cells
[0055] The tumor cells were inoculated on a cell culture dish, and 10 ml of 1640 medium containing 10% fetal bovine serum and 1% double antibody (penicillin-streptomycin, 10000 units / ml) was added. The dishes were incubated in a humidified incubator at 37°C containing 5% carbon dioxide. After a period of time, observe under the microscope, and when the cells are full, digest the cells from the culture dish with trypsin, collect them by centrifuging at a speed of 500g for 5 minutes, and wash them three times with PBS. Record the number of cells with a cell counter. The collected cells were treated with 20% ethanol solution at 4° C. for 15 minutes to inactivate tumor cells, and were centrifuged at 500 g for 10 minutes to collect the cells. Redisperse the cells with PBS, centrifuge at 500g for 10 minutes, repeat the above PBS dispersion and centrifugation three...
Embodiment 2
[0064] [Example 2] Preparation of Nano Cell Membrane Tumor Vaccines (NP@FMs)
[0065] The preparation of inactivated tumor cells and BMDCs in this example is derived from Example 1.
[0066] 1. Fusion inactivated tumor cells and dendritic cells to obtain fusion cells;
[0067] The dendritic cells dispersed in PBS and the inactivated tumor cells dispersed in PBS were evenly mixed at a ratio of 10:1, centrifuged at 500 g for 10 minutes, and the supernatant was discarded as much as possible. Within 60 seconds, 2 ml of serum-free 1640 medium containing 50% polyethylene glycol (molecular weight: 2000) and 10% dimethyl sulfoxide that had been warmed to 40° C. was added dropwise. Incubate at 40°C for 1 minute, slowly add serum-free 1640 medium to 50 ml, and terminate the fusion process. Centrifuge at a speed of 500g for 10 minutes to separate the cells, and then disperse the cells in a medium containing 10% fetal bovine serum, 1% double antibody (penicillin-streptomycin, 10000 units / ...
Embodiment 3
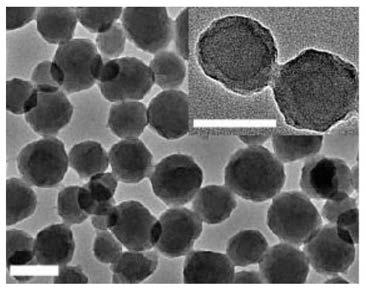
[0071] 120 mg of tetrakis (4-carboxyphenyl) porphin, 360 mg of ZrOCl 2 , and 3.36 g of benzoic acid were dissolved in 60 ml of N,N-dimethylformamide. Stirring at 90° C. for 10 hours, the mixed solution was centrifuged at 12000 rpm for 30 minutes, and washed three times with N,N-dimethylformamide to remove unreacted raw materials. The obtained NPs were stored in N,N-dimethylformamide solution. Before coating, replace N,N-dimethylformamide with ultrapure water by centrifugation (12000rpm for 30 minutes). Mix 10 mg / mL of confluent cell membranes and 10 mg / mL of nanoparticles, and sonicate the mixed solution for 1 min in an ice-water bath. The mixed solution is centrifuged at a speed of 10,000 g for 30 minutes to obtain the nano-cell membrane tumor vaccine. [Example 3] Preparation of Nano Cell Membrane Tumor Vaccines (NP@FMs)
[0072] The preparation of inactivated tumor cells and BMDCs in this example is derived from Example 1.
[0073] 1. Fusion inactivated tumor cells and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com