Humanized monoclonal antibody targeting Claudin18.2 as well as preparation method and application thereof
A technology of monoclonal antibody and humanization, applied in chemical instruments and methods, antibodies, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve problems such as side effects, and achieve simple preparation steps , the effect of good ADCC and CDC activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Example 1: Antigen Preparation
[0135] According to the sequence information of Claudin18.2, the gene was synthesized after codon optimization, and subcloned into the adenovirus shuttle vector pShuttle-CMV-GFP. Recombination to obtain the recombinant adenovirus plasmid.
[0136] The recombinant adenovirus vector was purified, and then transfected into 293A cells using LVTransm transfection reagent to prepare adenovirus seeds. Based on the seed virus, large-scale amplification is carried out, the adenovirus is purified, and the titer is determined using Anti-Hexon antibody.
Embodiment 2
[0137] Example 2: Mouse Immunization
[0138] All mice were housed in a barrier system with sterile pelleted chow and autoclaved drinking water. Take 8 Balb / c mice (SPF grade) and mark them with ear tags, and follow the classic immunization process, using purified recombinant adenovirus, according to 1×10 7 pfu / mouse dosage, mice were immunized by intramuscular injection.
[0139] Table 1 Claudin18.2 recombinant adenovirus animal immunization scheme
[0140]
[0141]
Embodiment 3
[0142] Example 3: Detection of mouse serum titer after immunization
[0143] 1. Take the mouse out of the cage, use 75% medical alcohol cotton balls to disinfect the tail of the mouse, and use a blood collection needle to prick a small wound on the tail of the mouse;
[0144] 2. Use capillary glass blood collection tubes to collect blood drops;
[0145] 3. After collecting blood, use a dry sterile cotton ball to gently press the blood collection point to stop the bleeding, and return the mouse to the cage for observation;
[0146] 4. Place the centrifuge tube with collected blood samples in a 37°C incubator for 1 hour; then transfer the blood samples to 4°C overnight.
[0147] 5. Separate the serum from the blood clot, transfer it to a new sterile centrifuge tube, and centrifuge at 10,000 g for 10 min at 4°C;
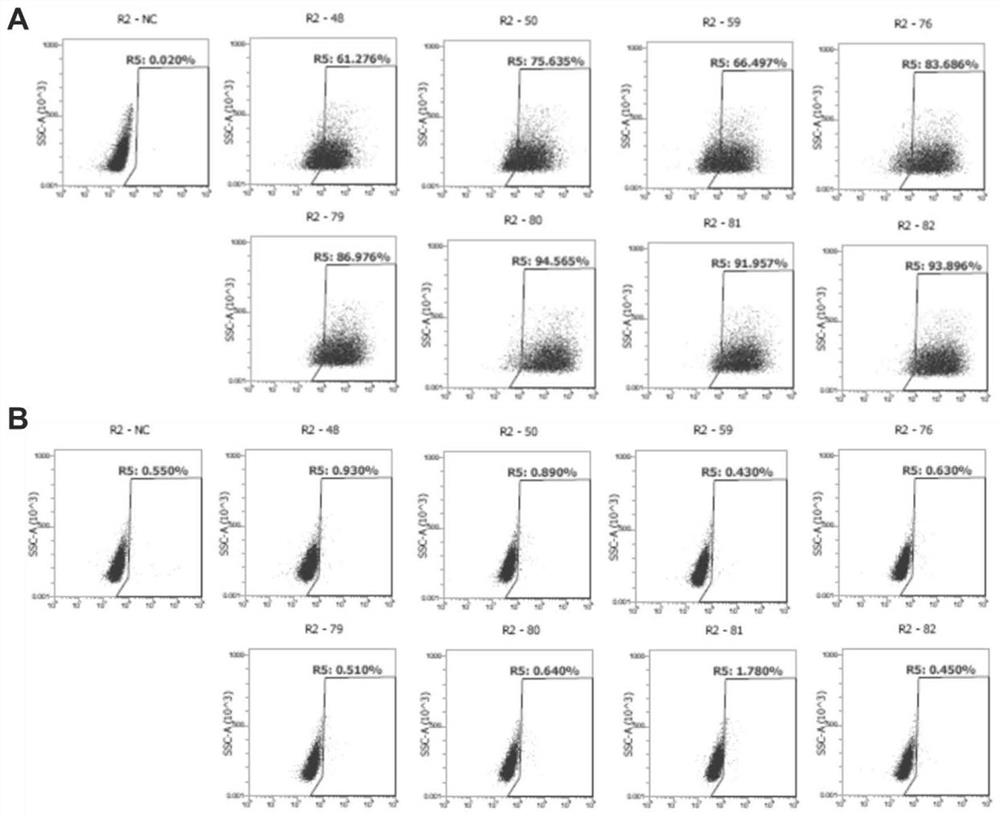
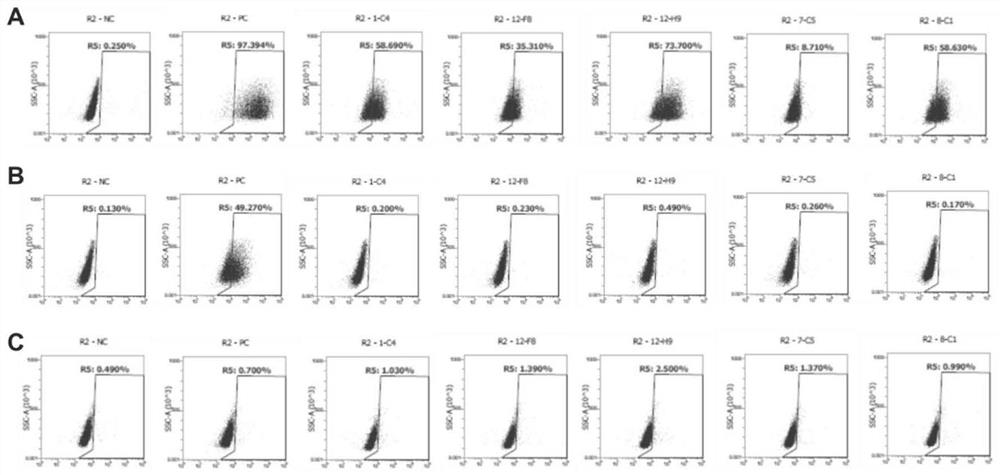
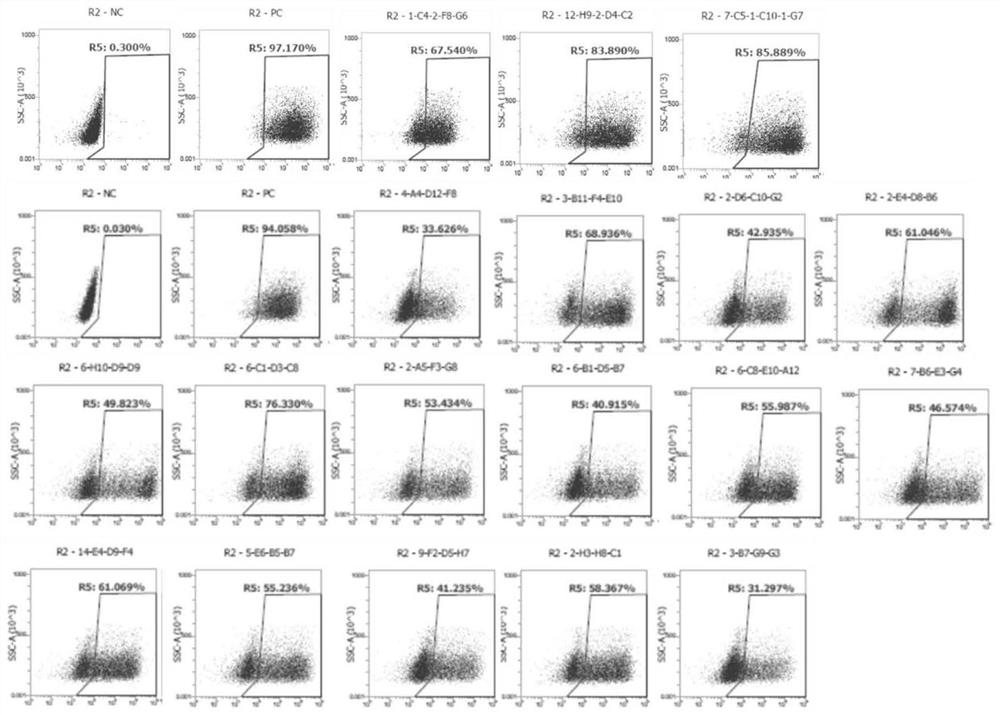
[0148] 6. Dilute the serum at 1:1000, and perform FACS detection with CHO-Claudin18.2 cells and control cell CHO respectively.
[0149] The results of titer detectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com