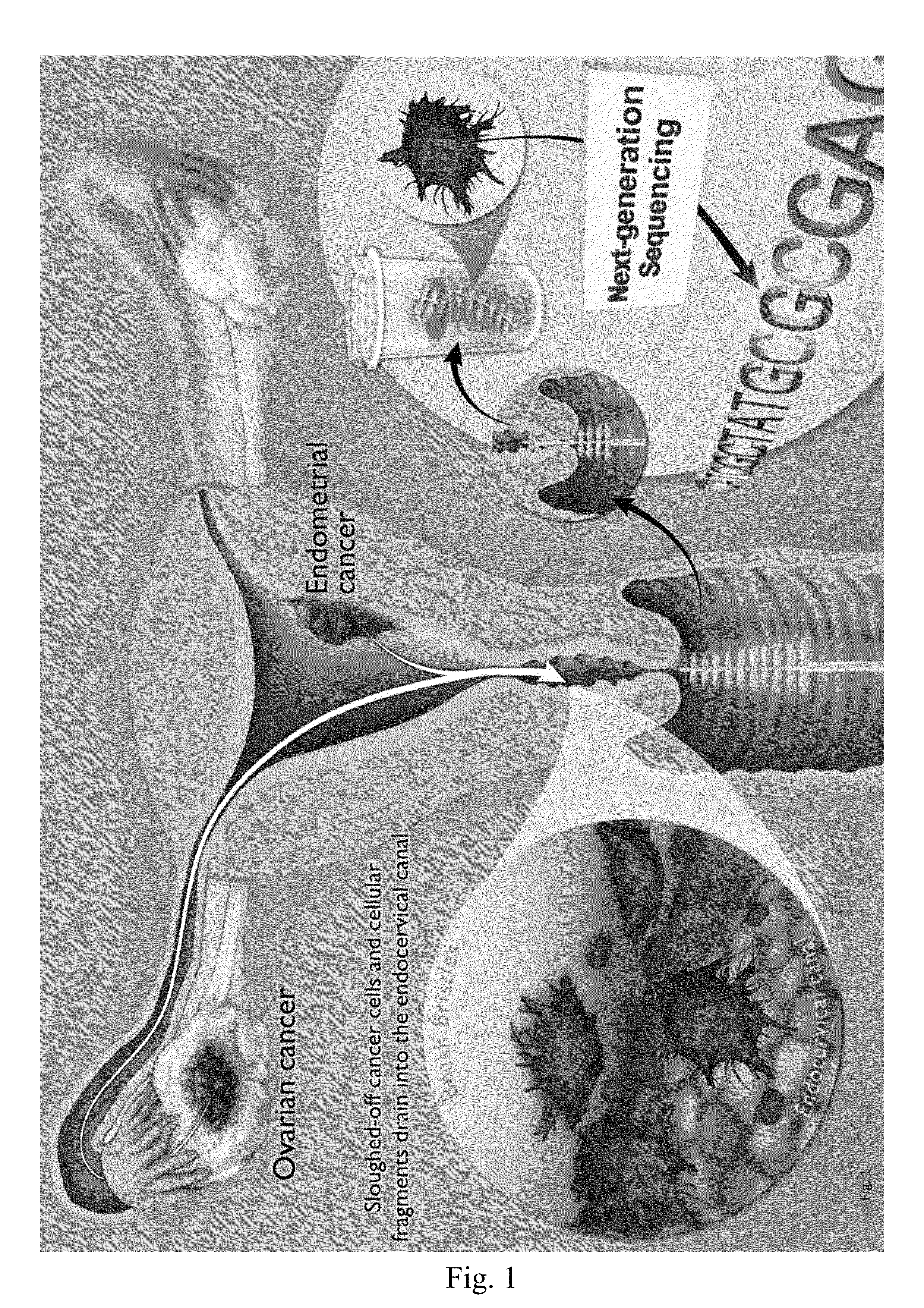Patents
Literature
34 results about "Pap smears" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
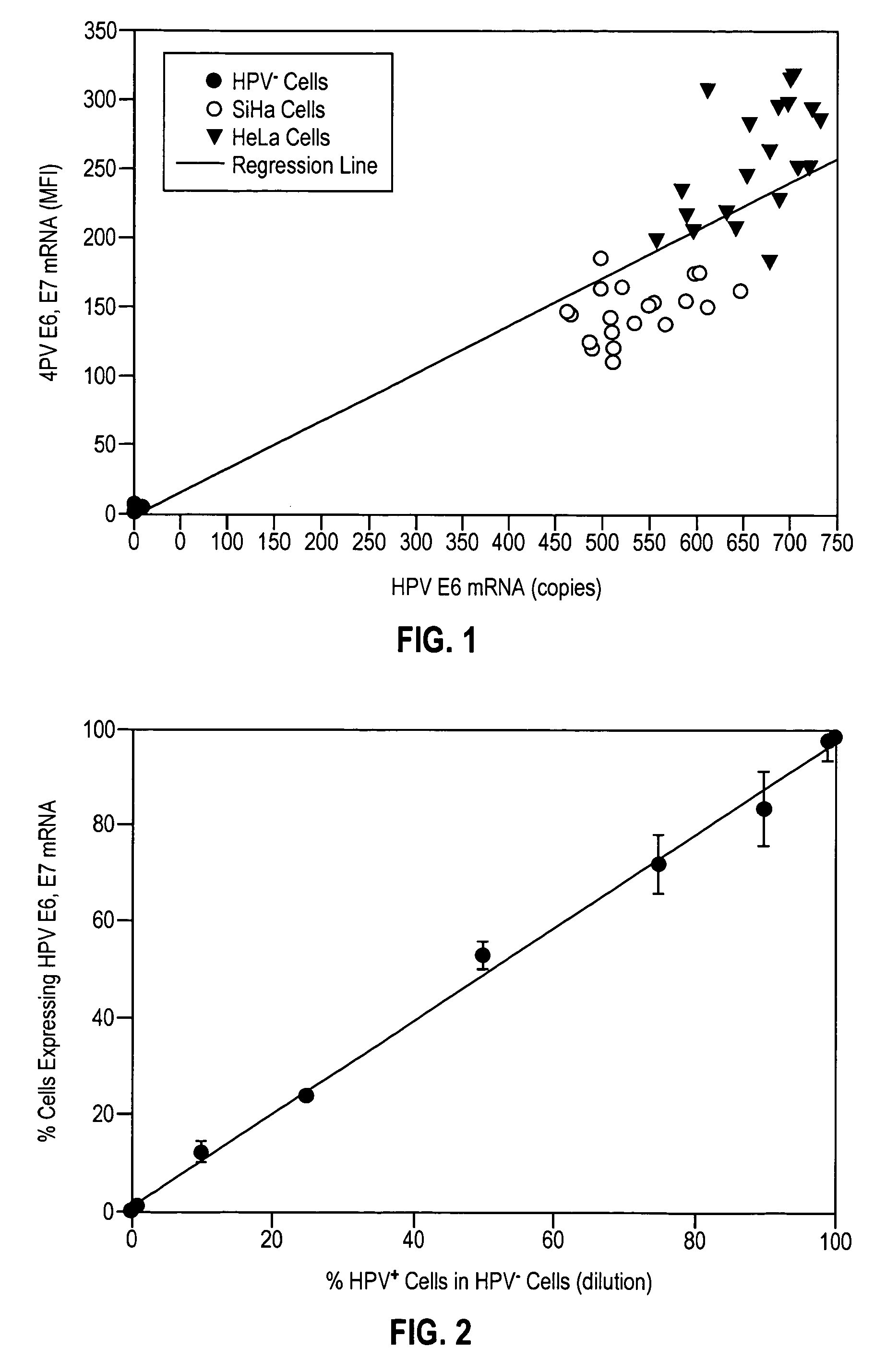
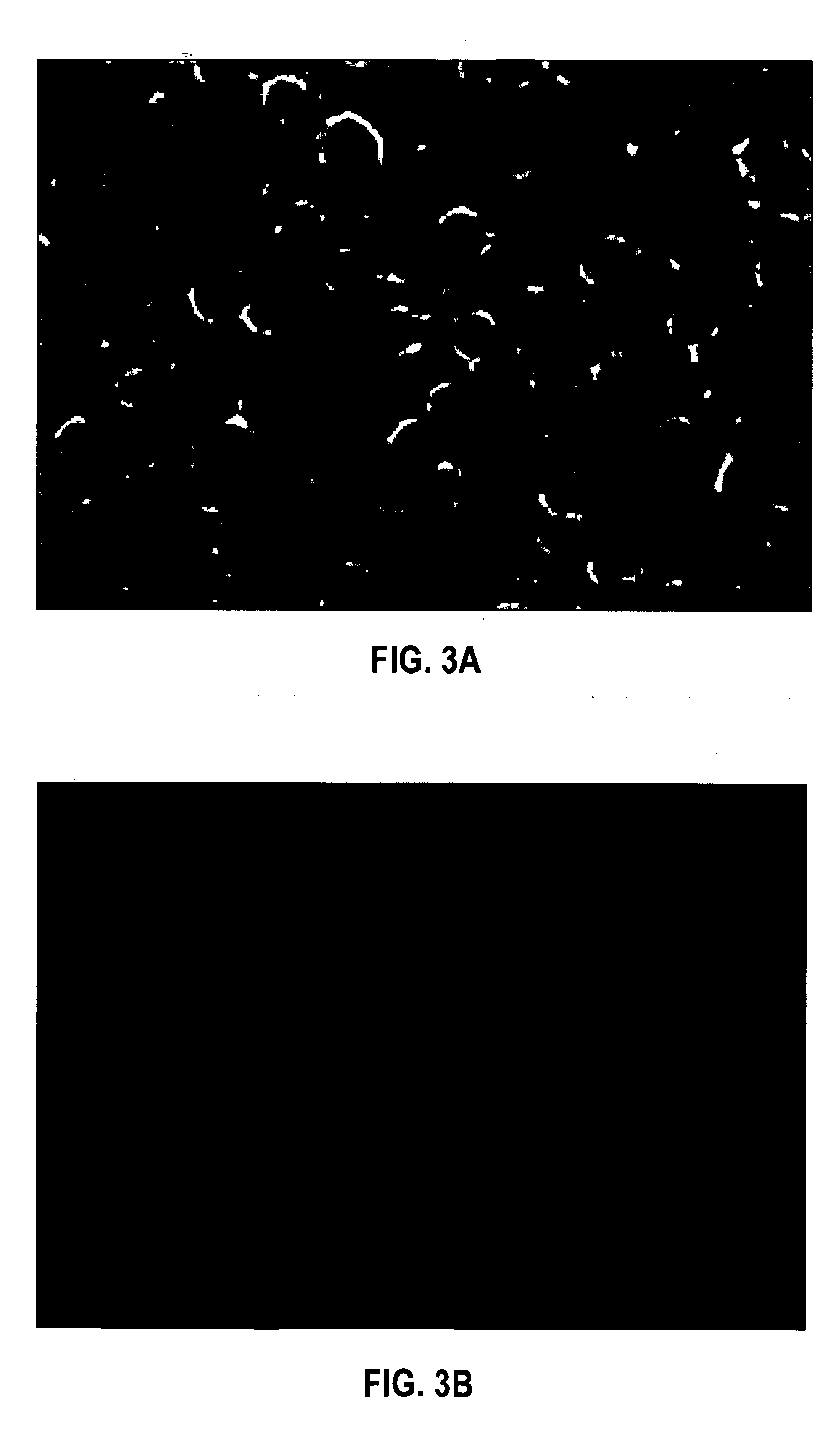
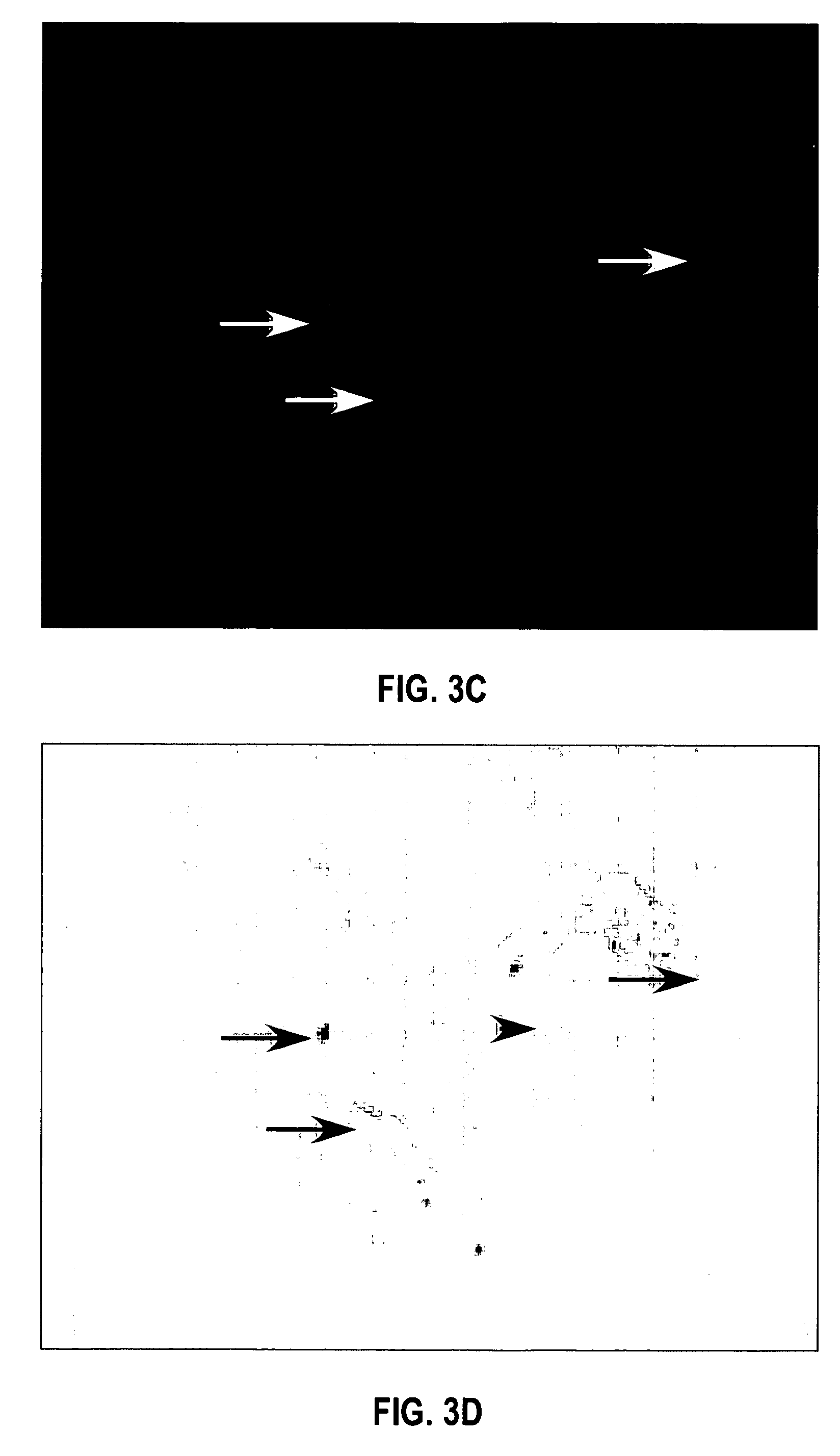
HPV E6, E7 mRNA assay and methods of use thereof
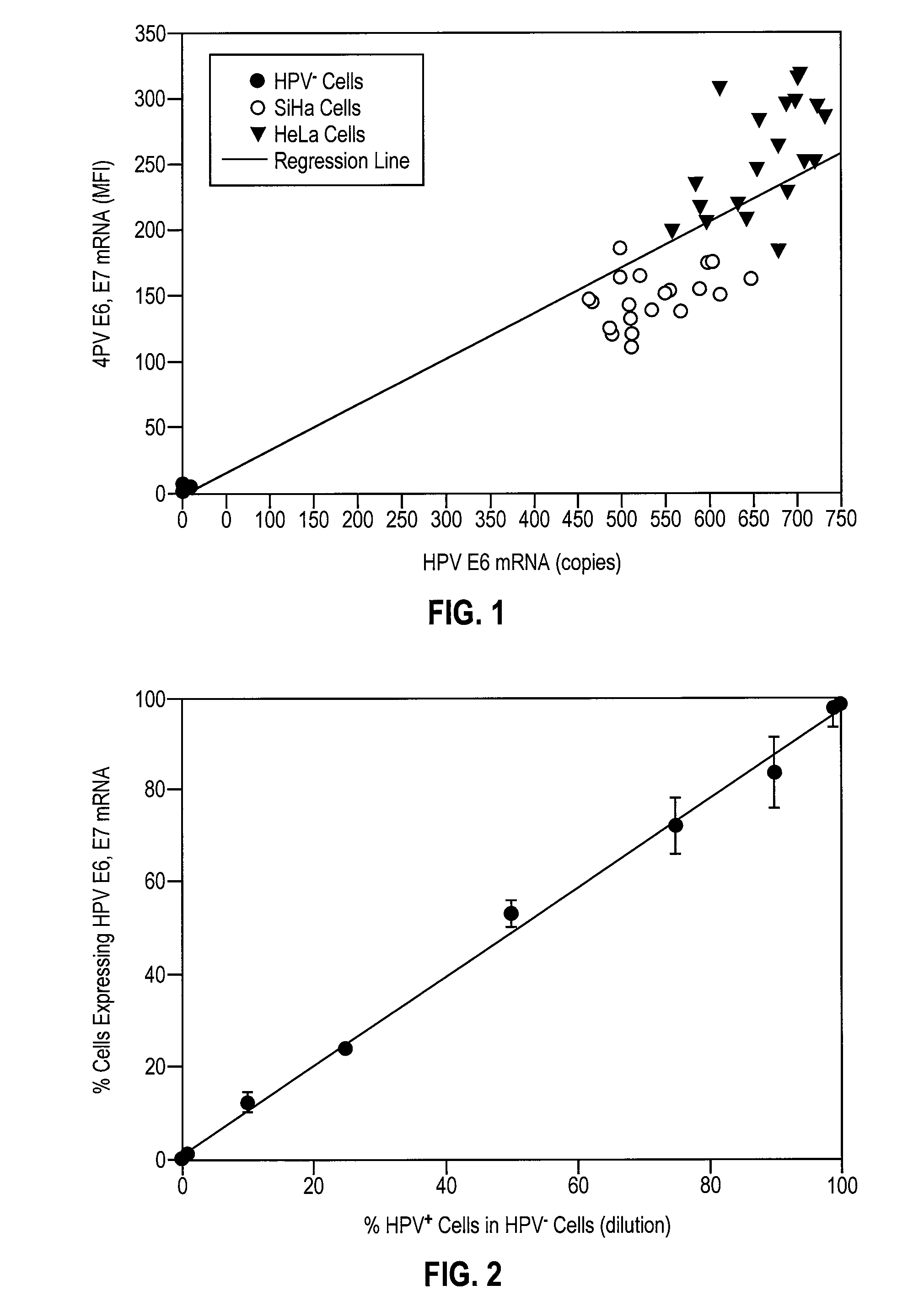
Provided is an HPV E6, E7 mRNA assay, referenced herein as the “In Cell HPV Assay,” that is capable of sensitive and specific detection of normal cervical cells undergoing malignant transformation as well as abnormal cervical cells with pre-malignant or malignant lesions. The In Cell HPV Assay identifies HPV E6, E7 mRNA via in situ hybridization with oligonucleotides specific for HPV E6, E7 mRNA and quantitates the HPV E6, E7 mRNA via flow cytometry. The In Cell HPV Assay can be carried out in less than three hours directly from liquid-based cervical (“LBC”) cytology specimens. The In Cell HPV Assay provides an efficient and highly sensitive alternative to the Pap smear for determining abnormal cervical cytology.
Owner:INCELLDX
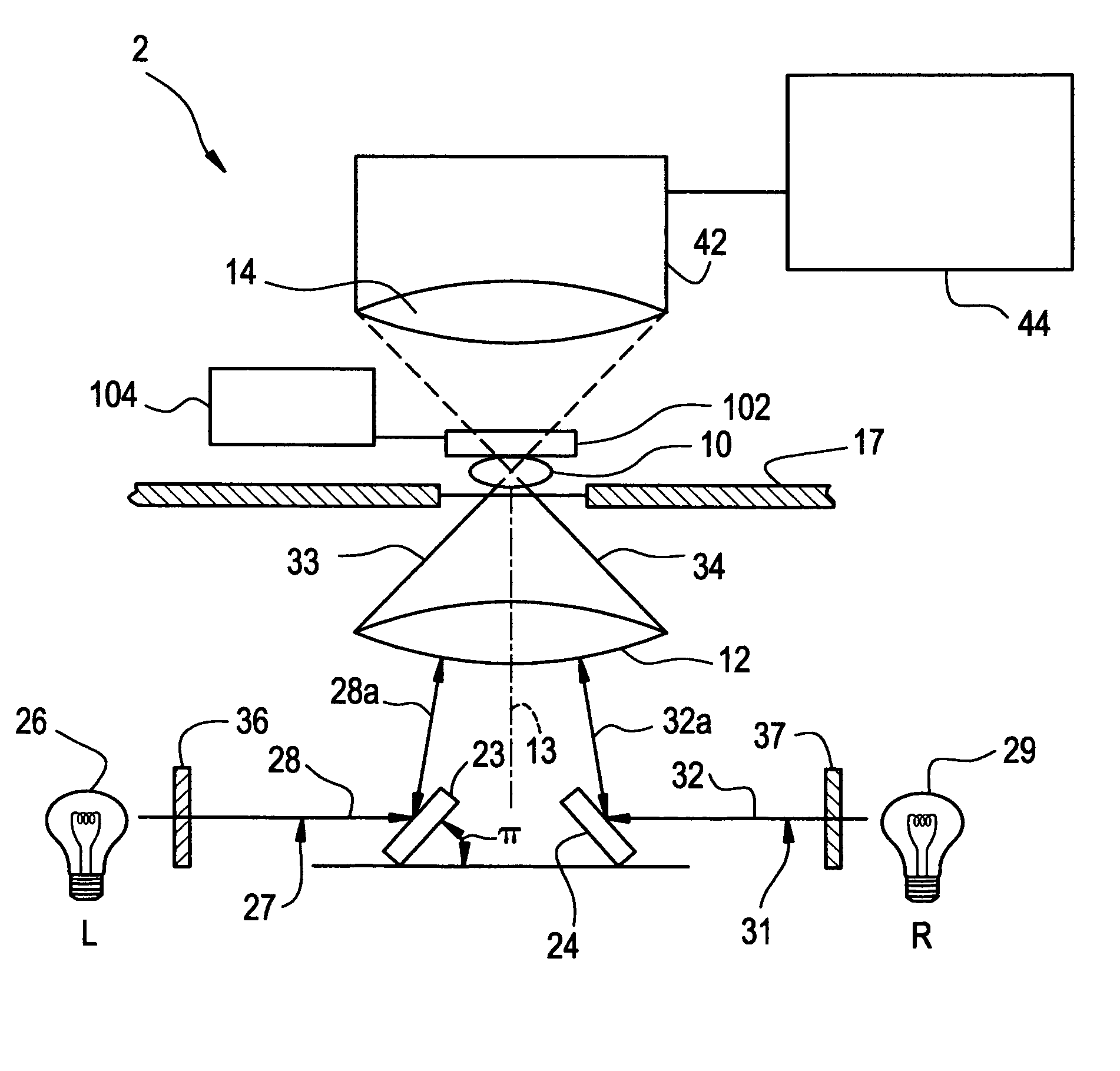
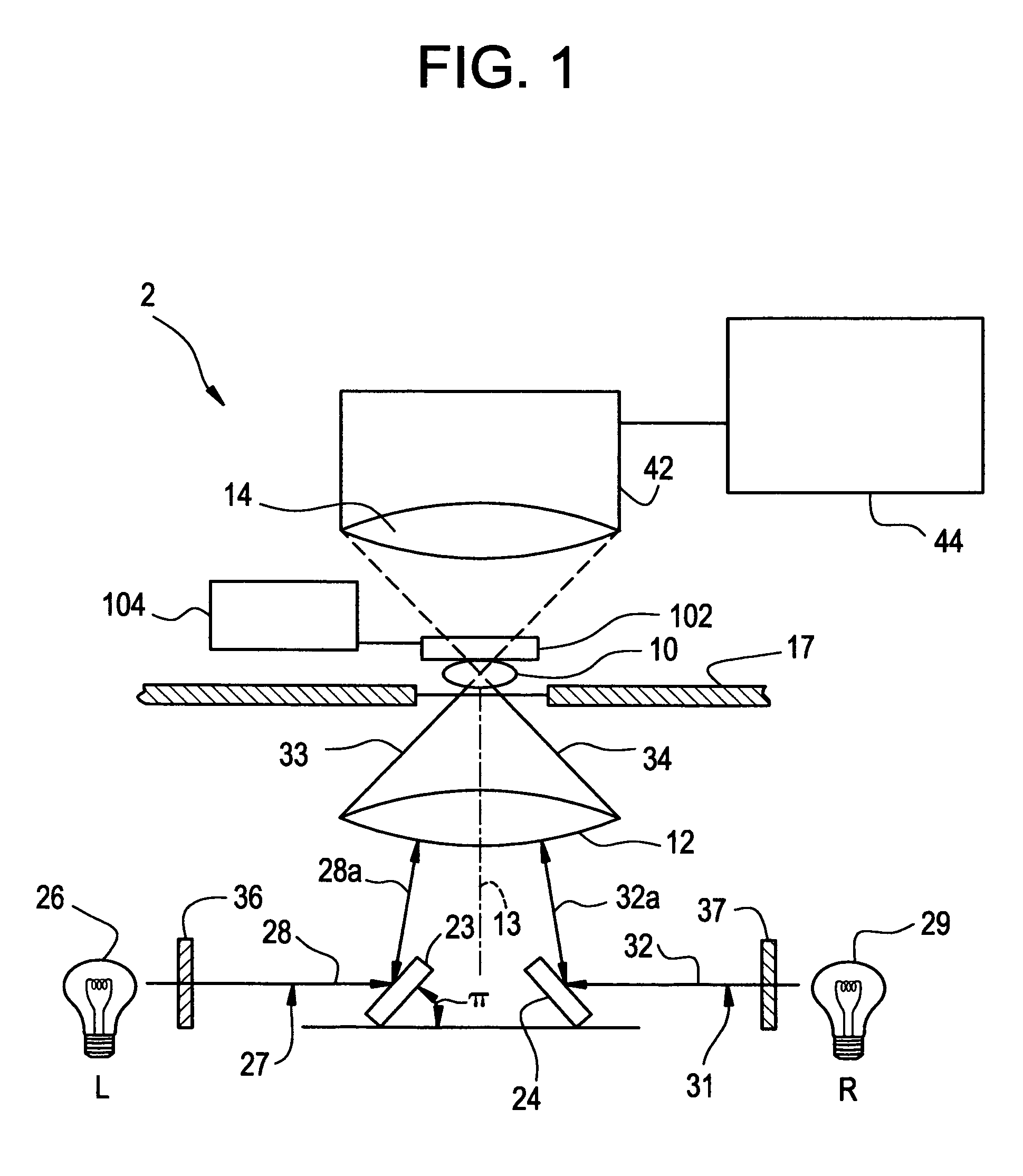
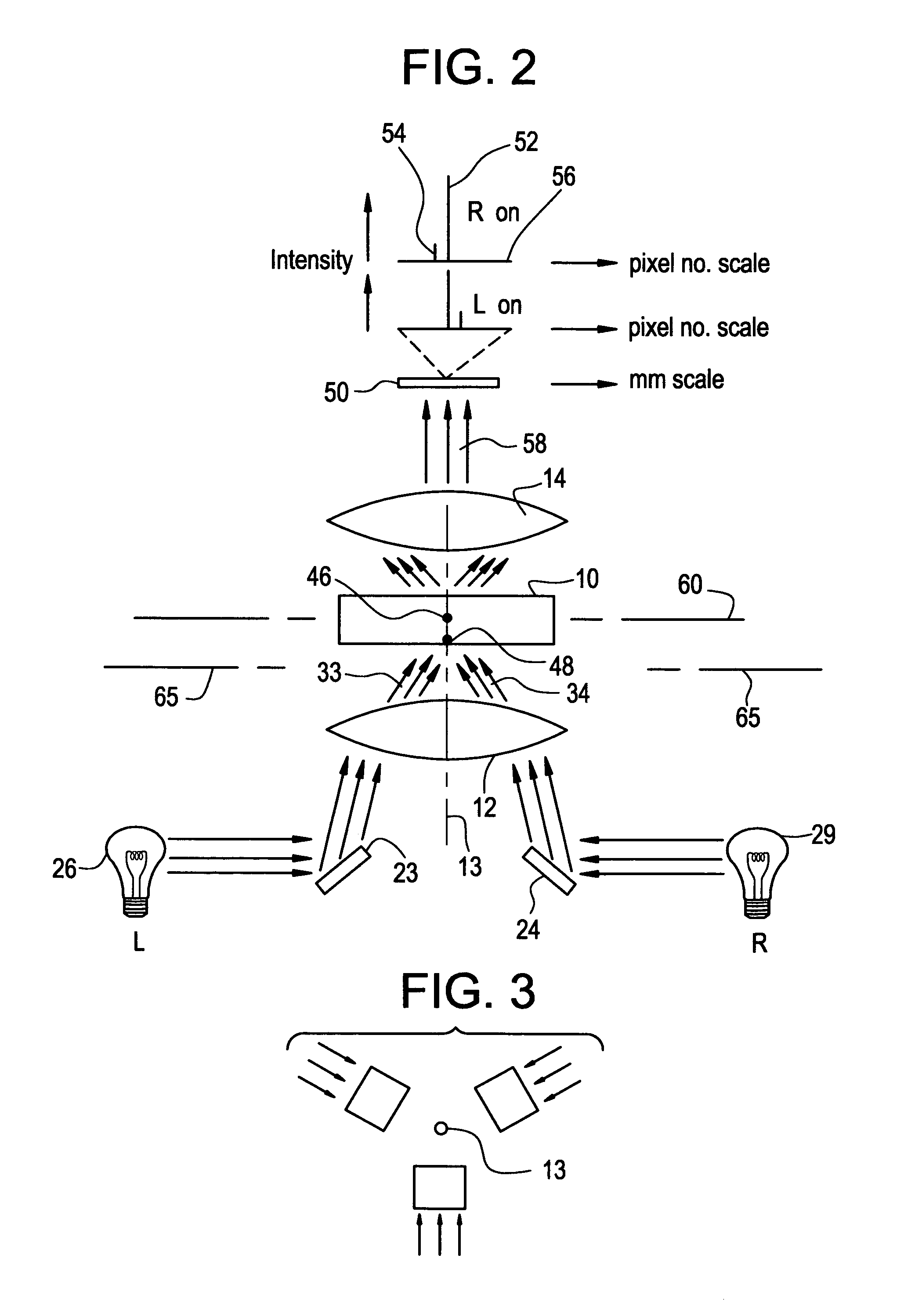
Tomographic microscope for high resolution imaging and method of analyzing specimens
InactiveUS7003143B1Eliminate undesirable informationEliminate informationLaser detailsMicroscopesHigh resolution imagingComputer science
Owner:HEWITT CHARLES W +4
HPV E6, E7 mRNA assay and methods of use thereof
Provided is an HPV E6, E7 mRNA assay, referenced herein as the “In Cell HPV Assay,” that is capable of sensitive and specific detection of normal cervical cells undergoing malignant transformation as well as abnormal cervical cells with pre-malignant or malignant lesions. The In Cell HPV Assay identifies HPV E6, E7 mRNA via in situ hybridization with oligonucleotides specific for HPV E6, E7 mRNA and quantitates the HPV E6, E7 mRNA via flow cytometry. The In Cell HPV Assay can be carried out in less than three hours directly from liquid-based cervical (“LBC”) cytology specimens. The In Cell HPV Assay provides an efficient and highly sensitive alternative to the Pap smear for determining abnormal cervical cytology.
Owner:INCELLDX
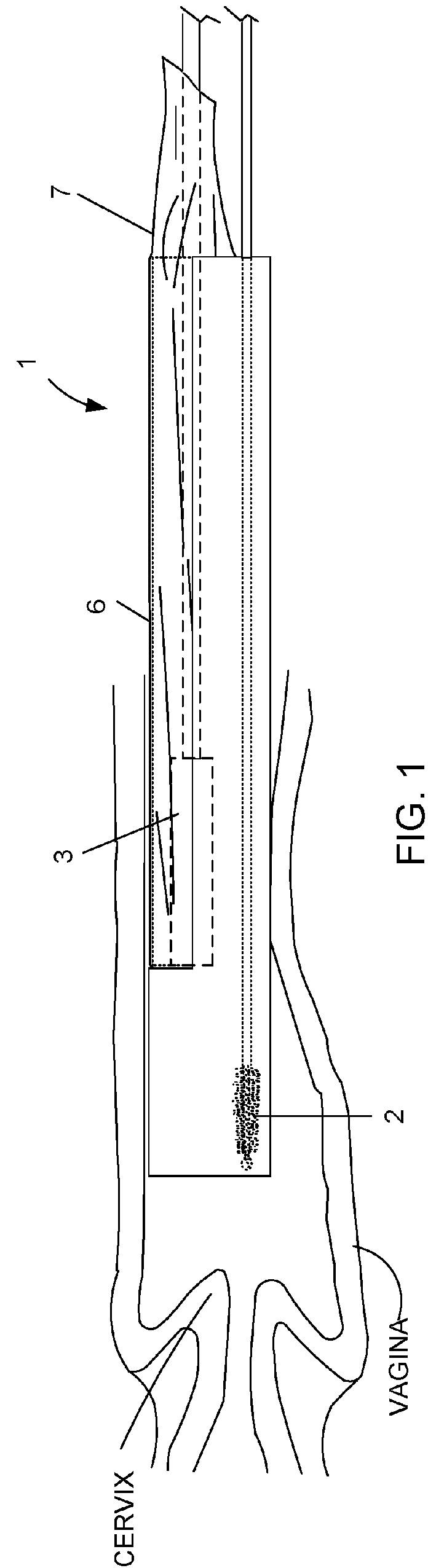
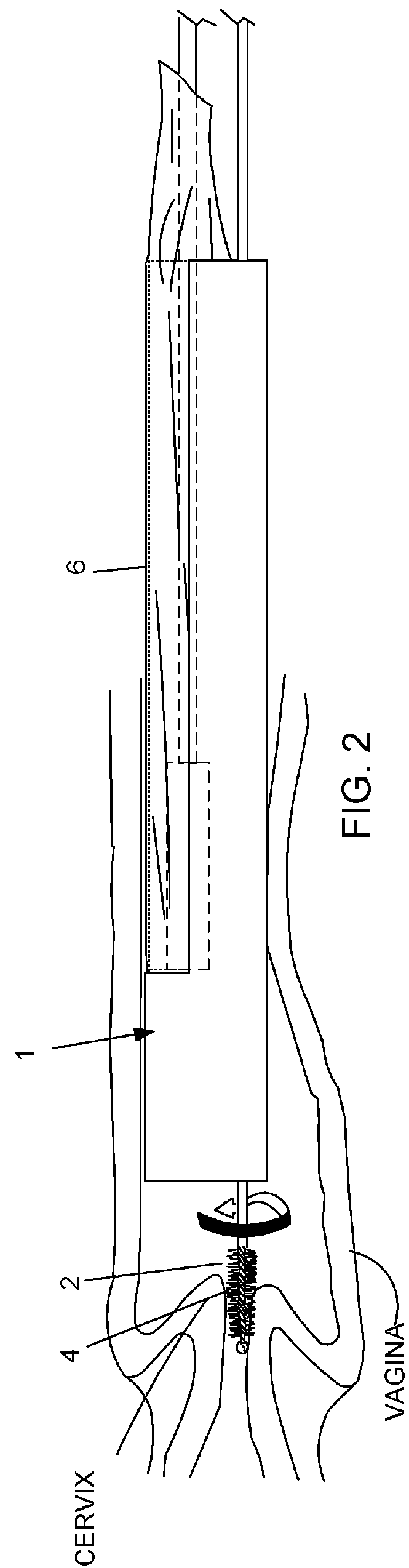
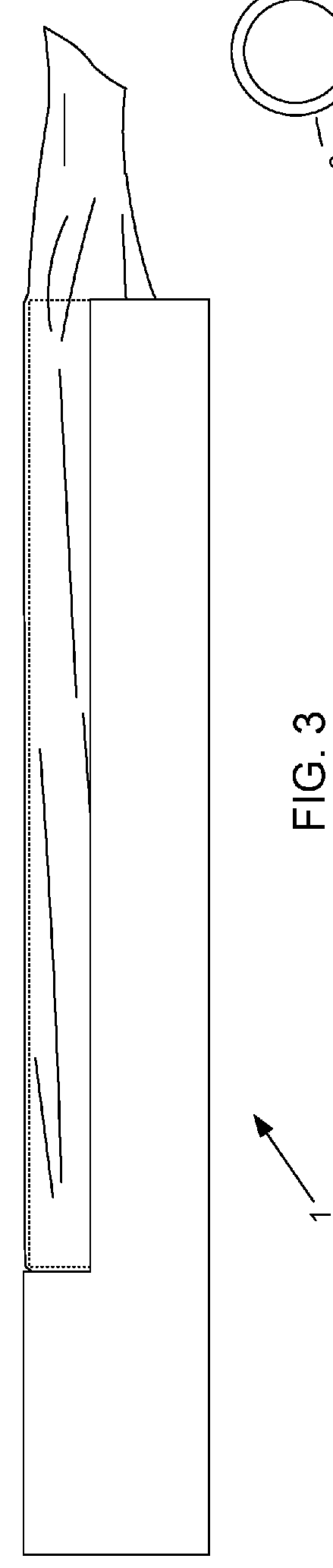
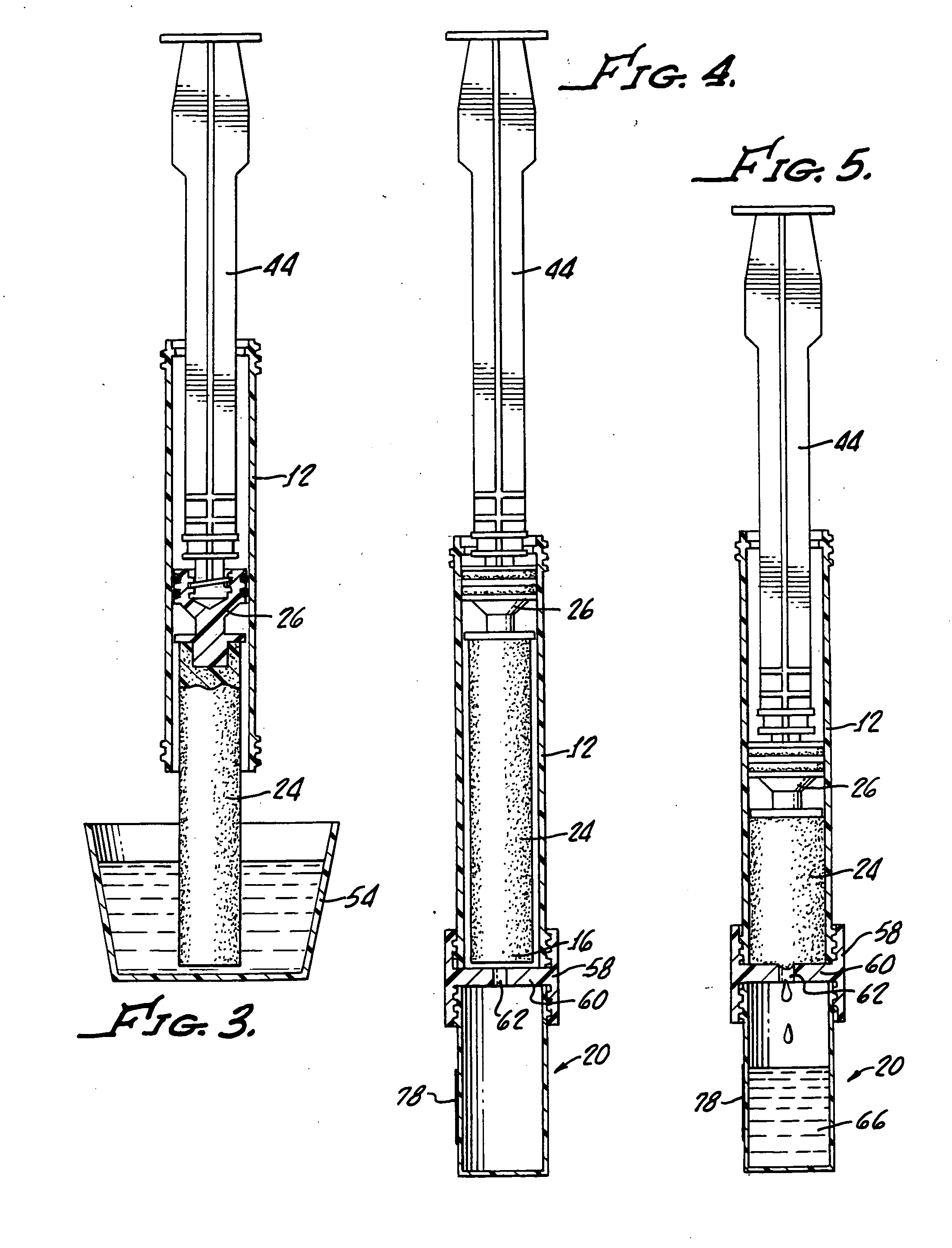
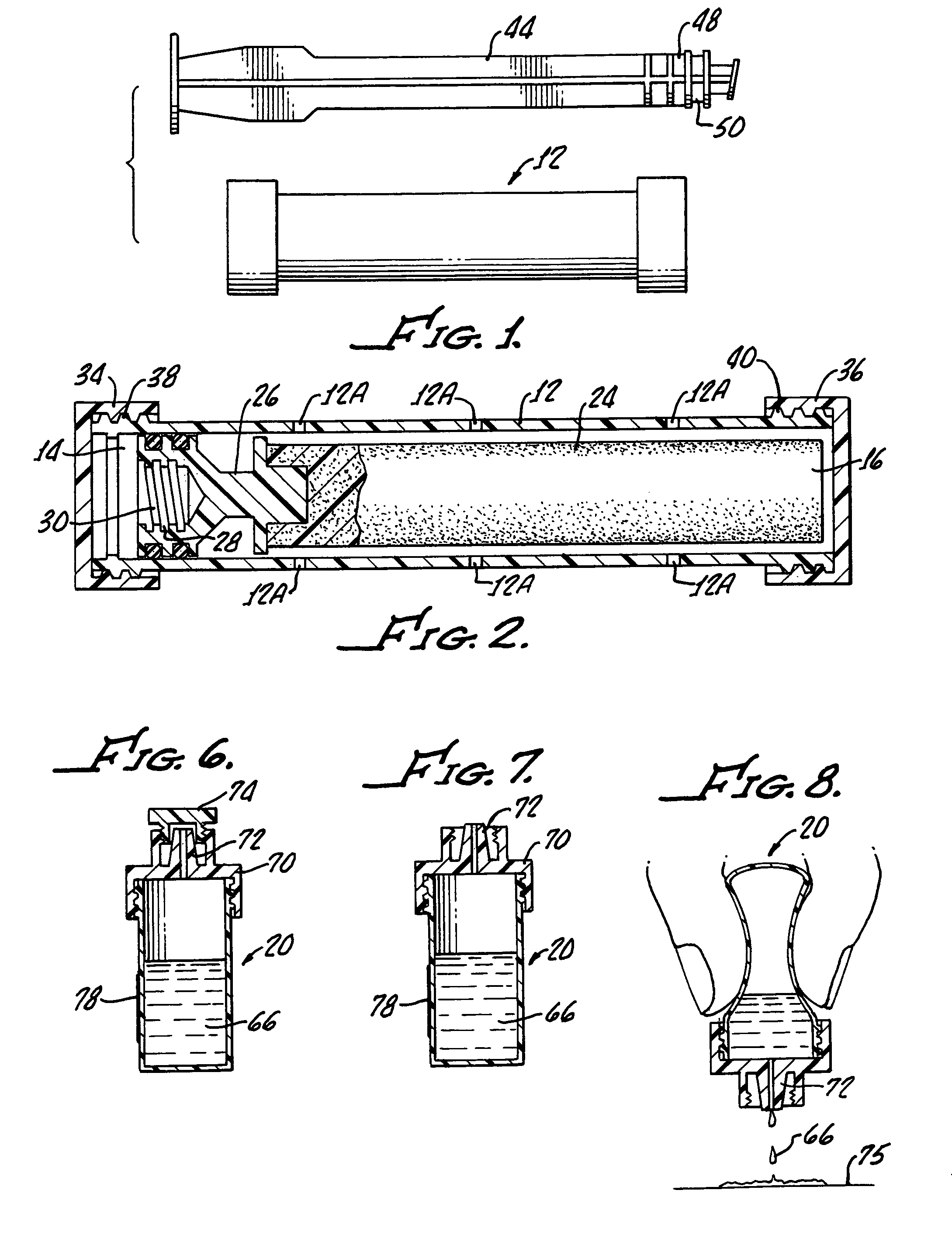
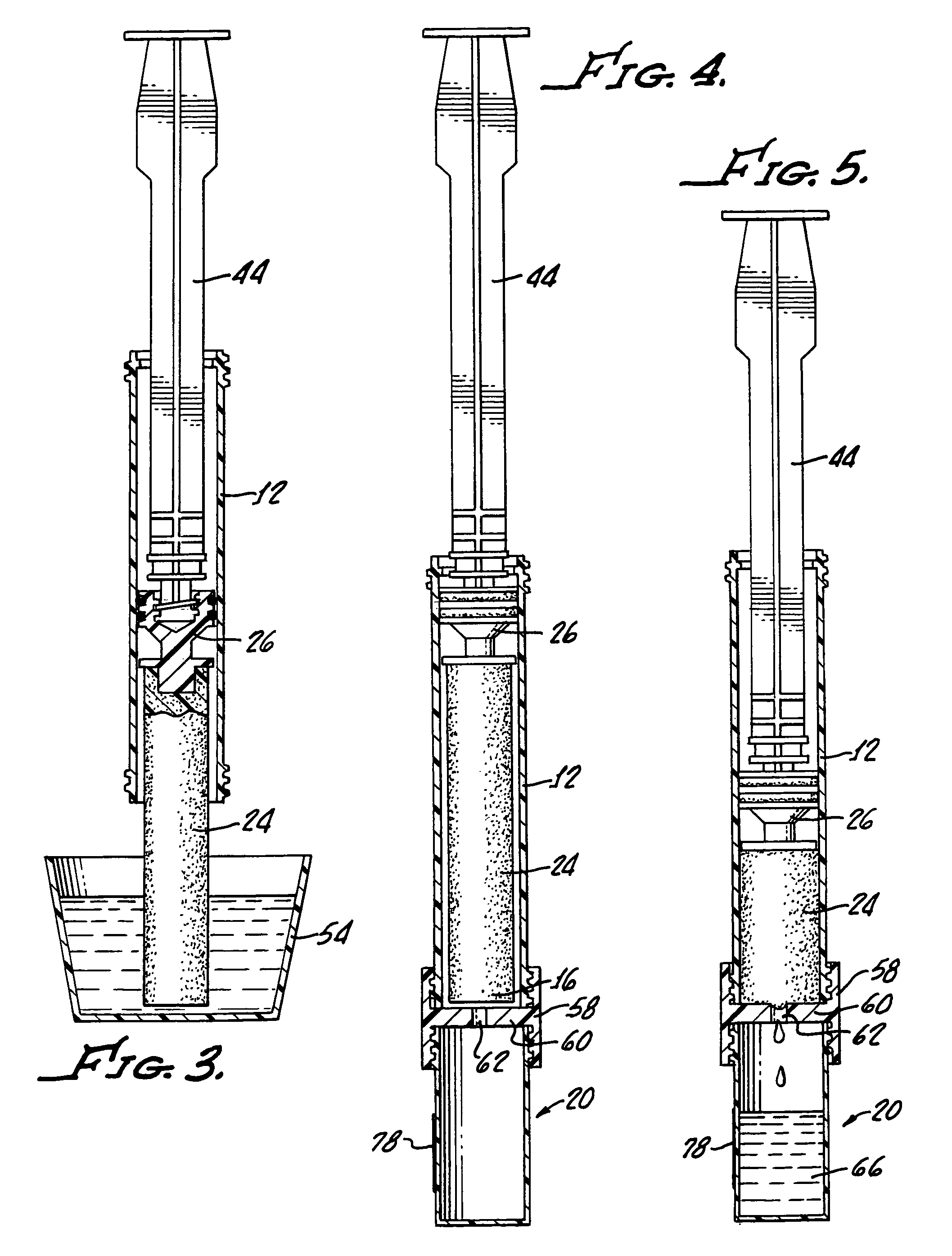
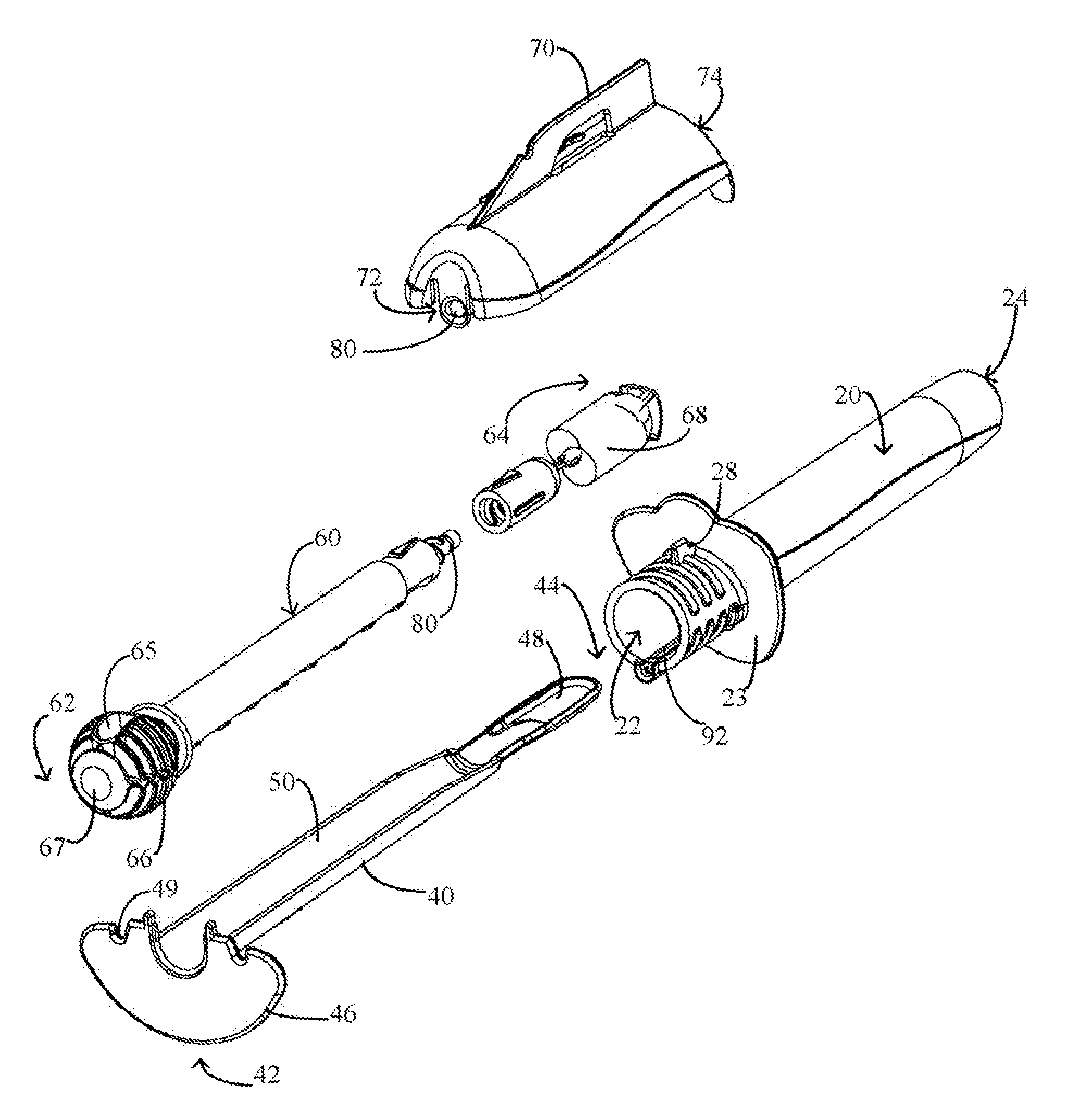
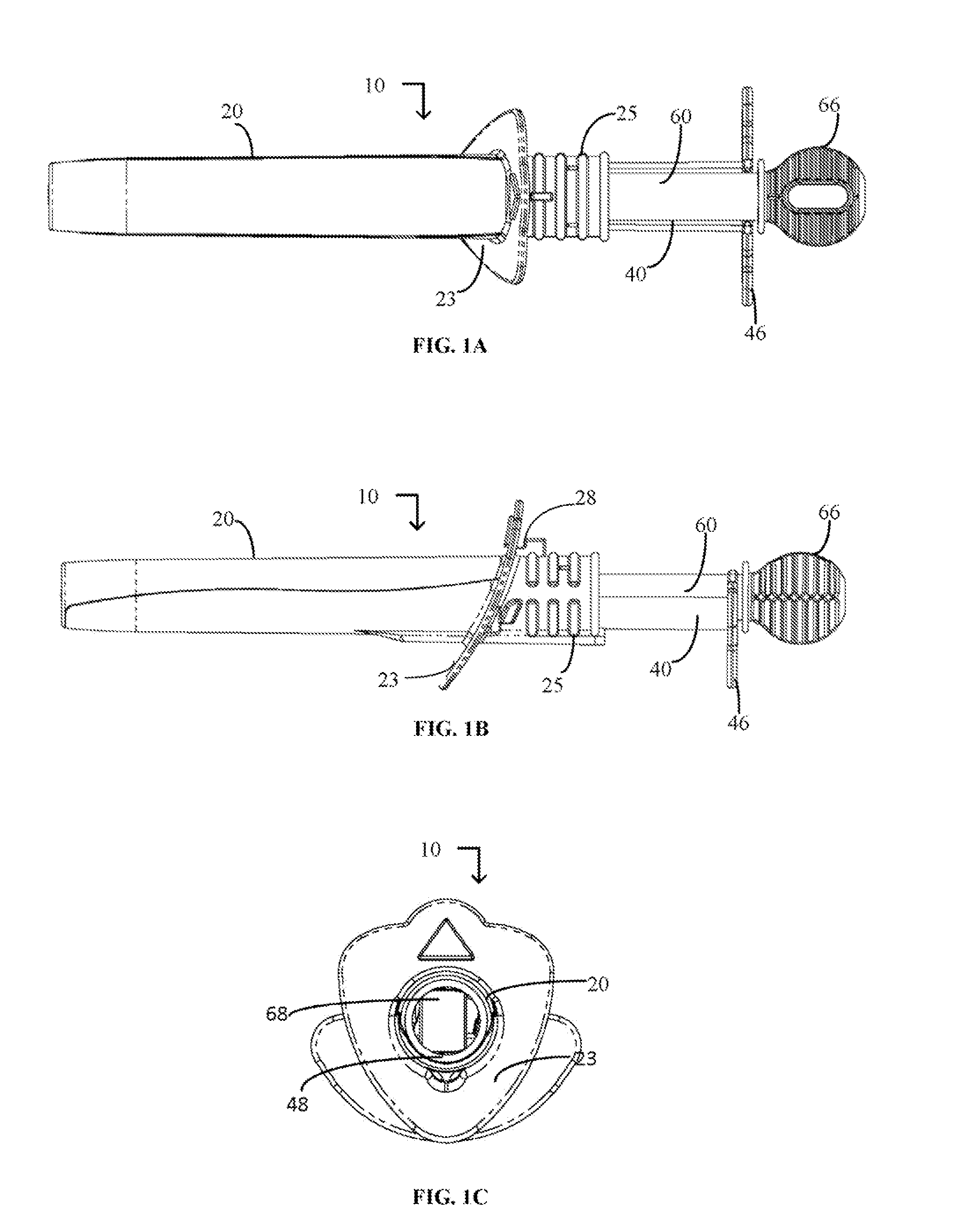
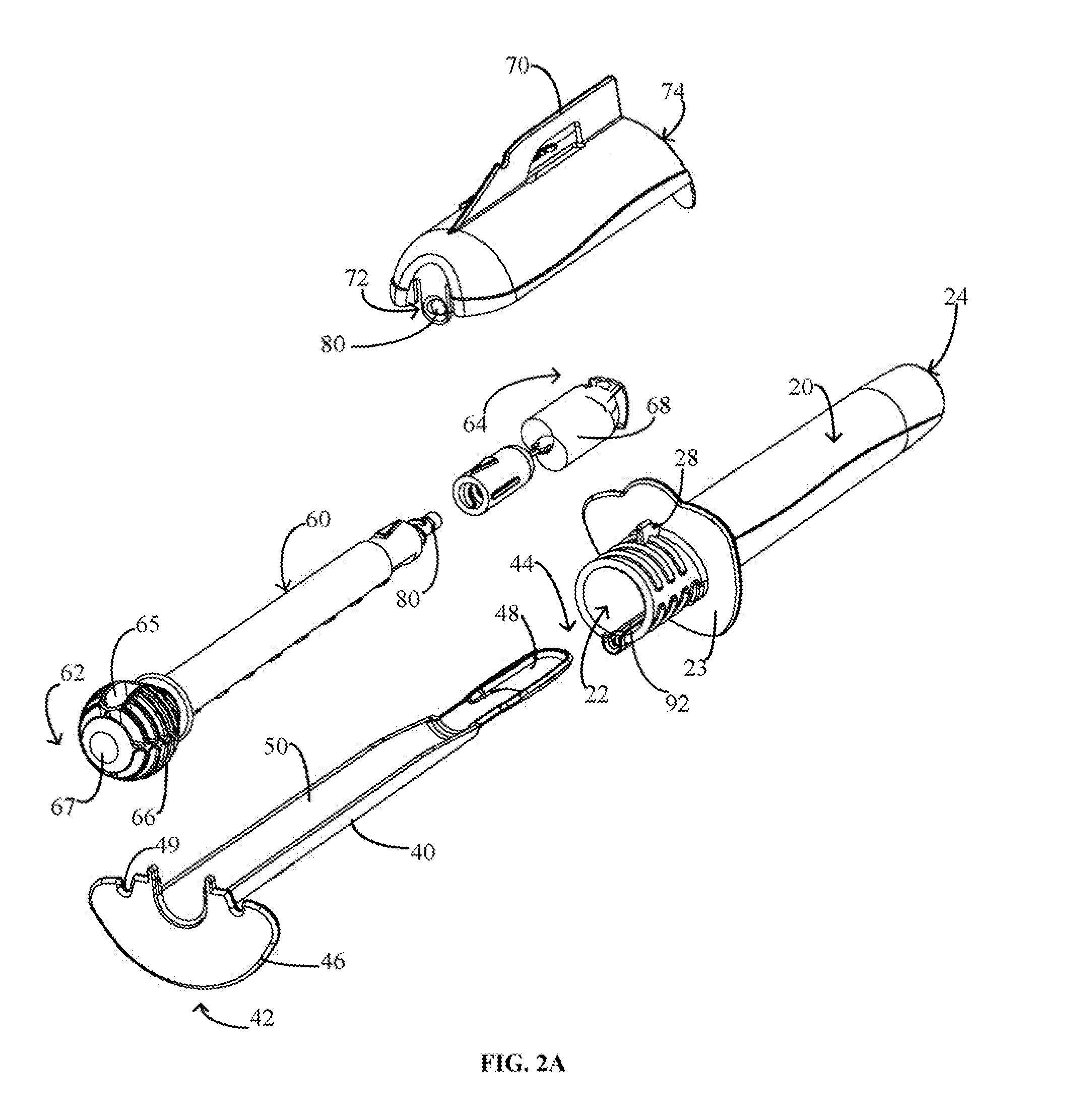
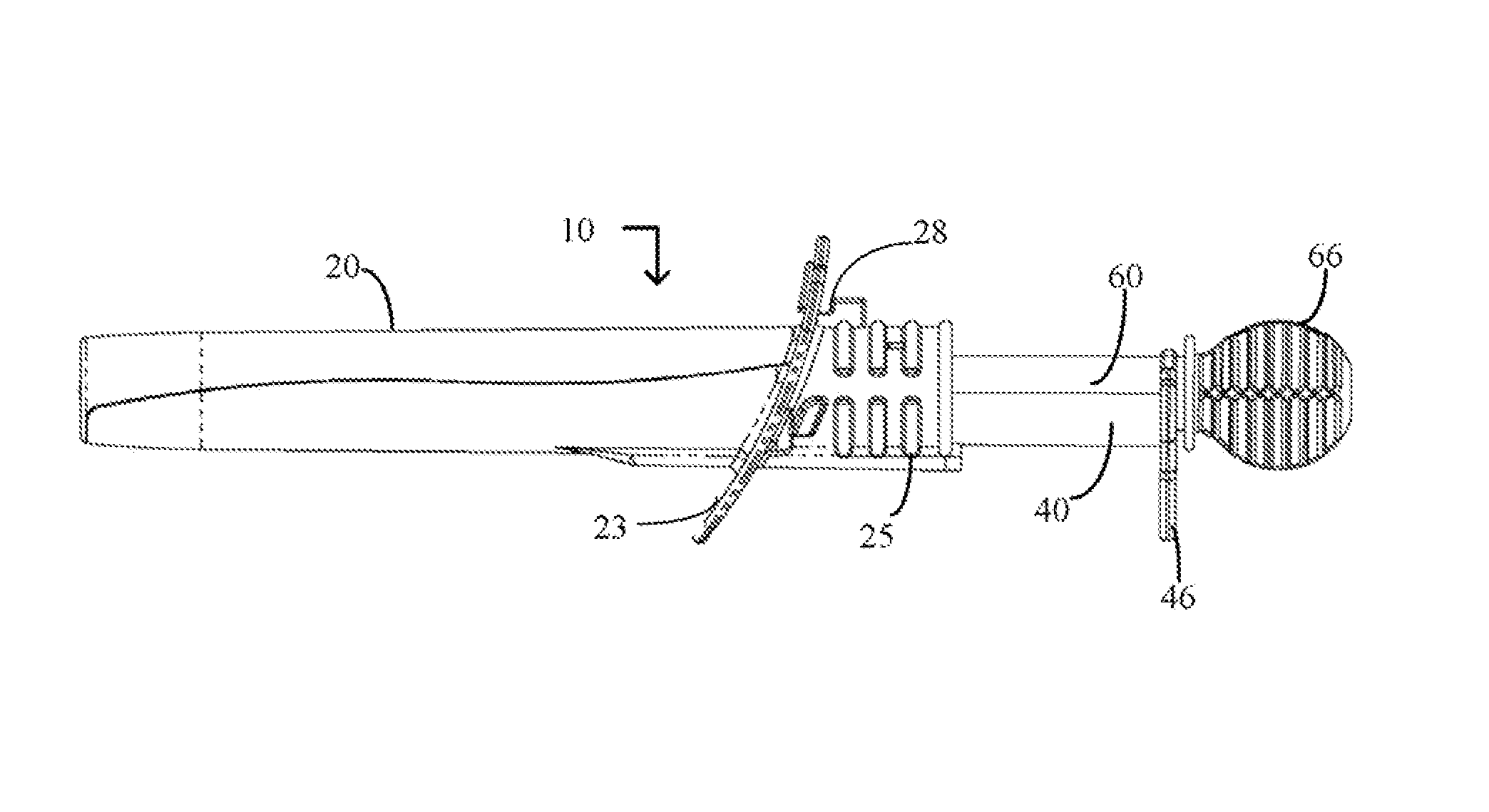
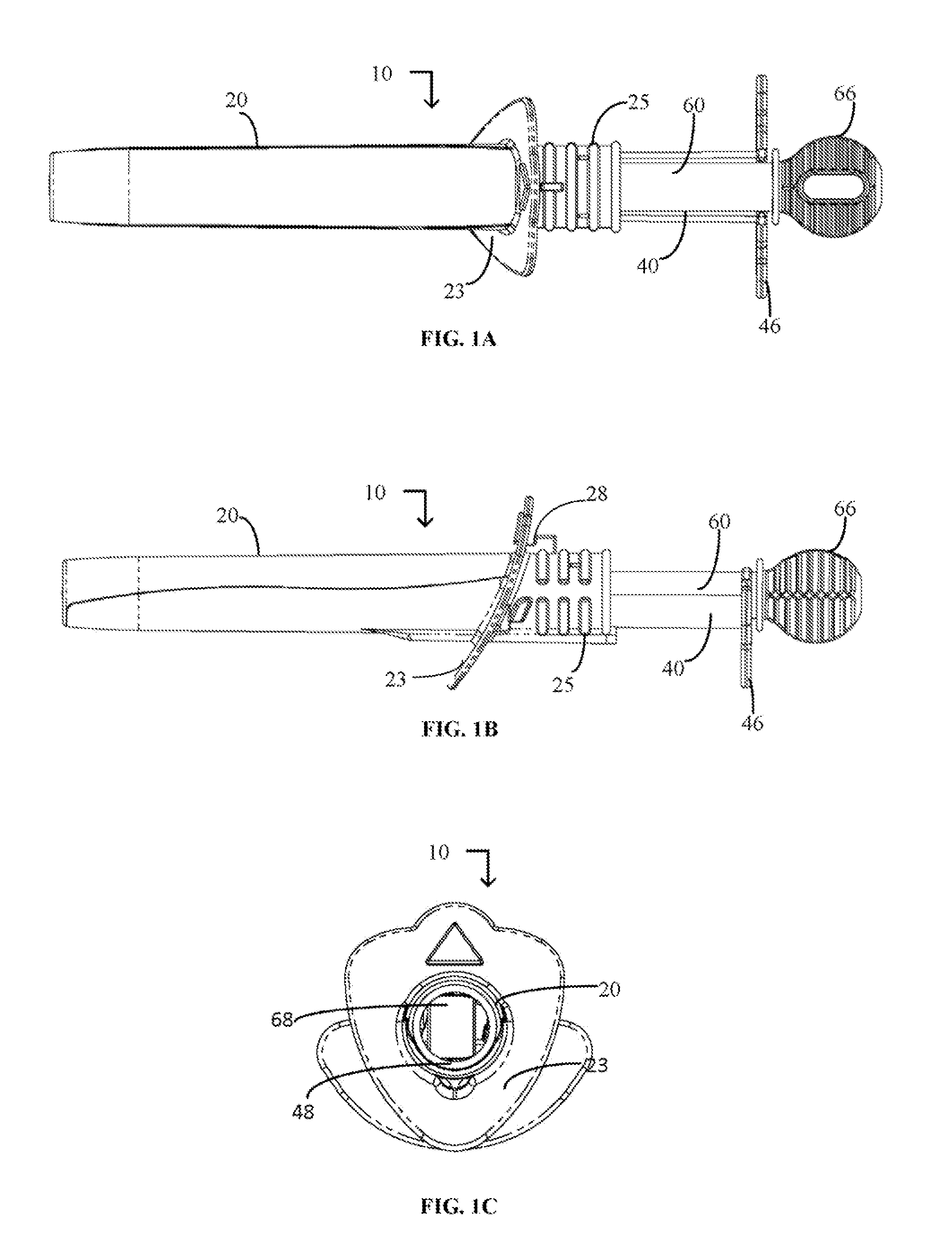
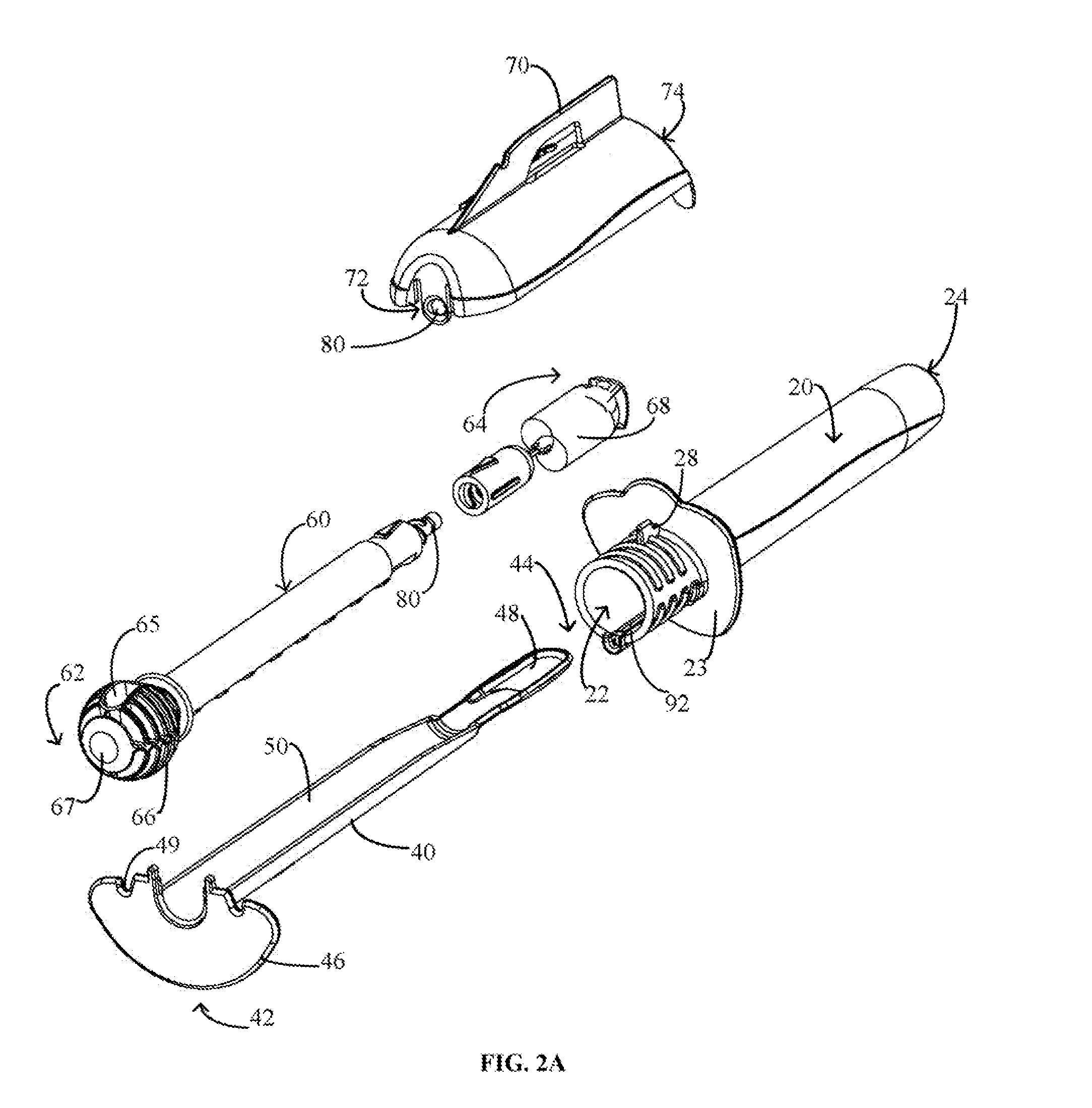
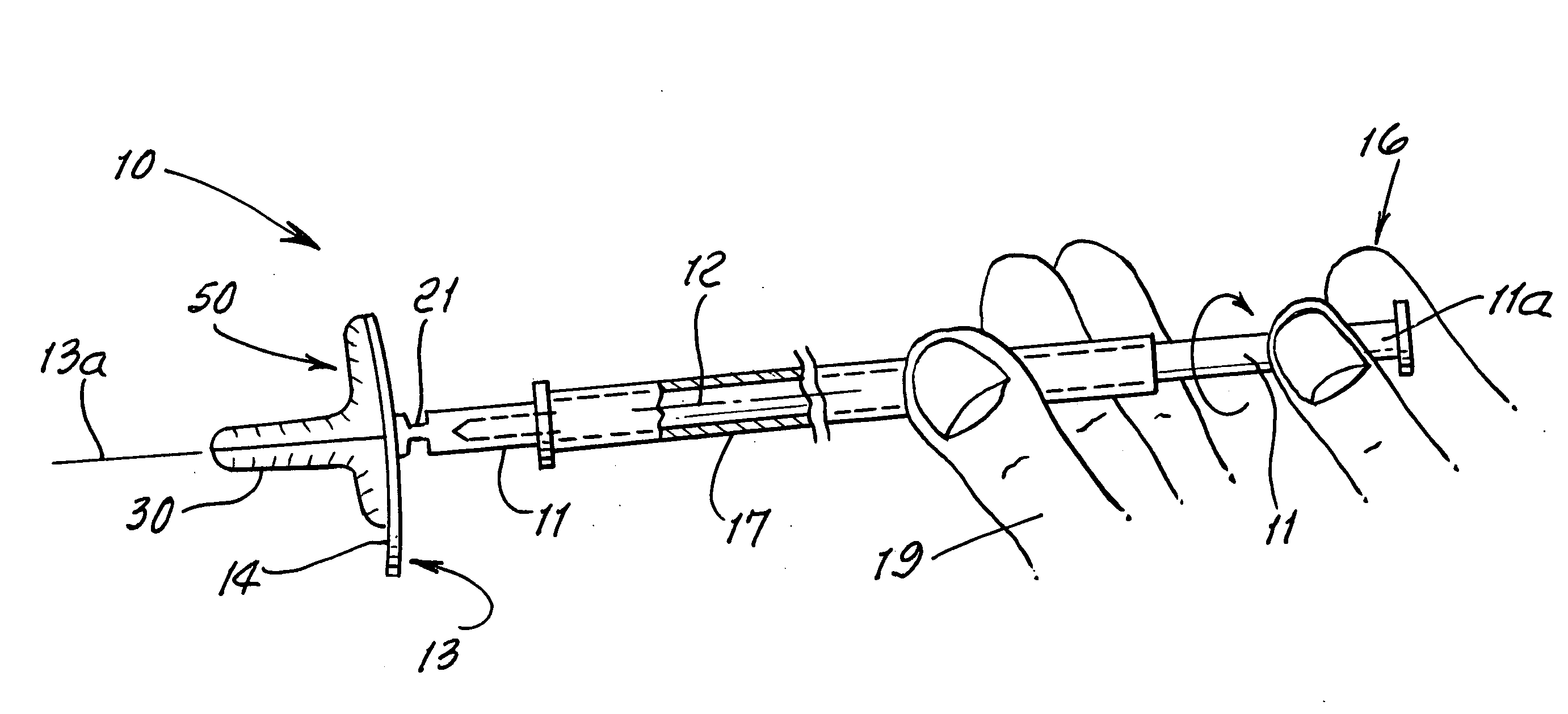
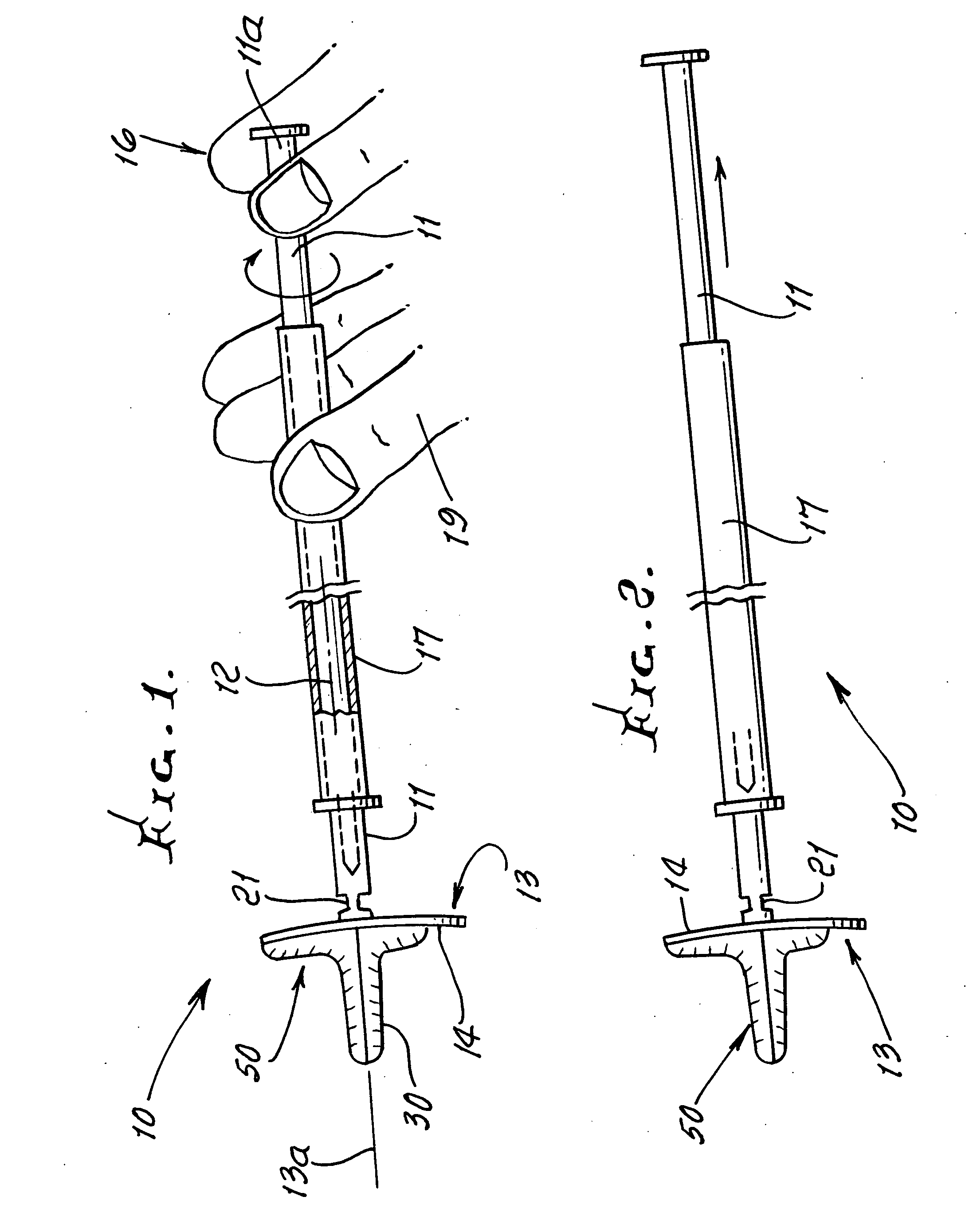
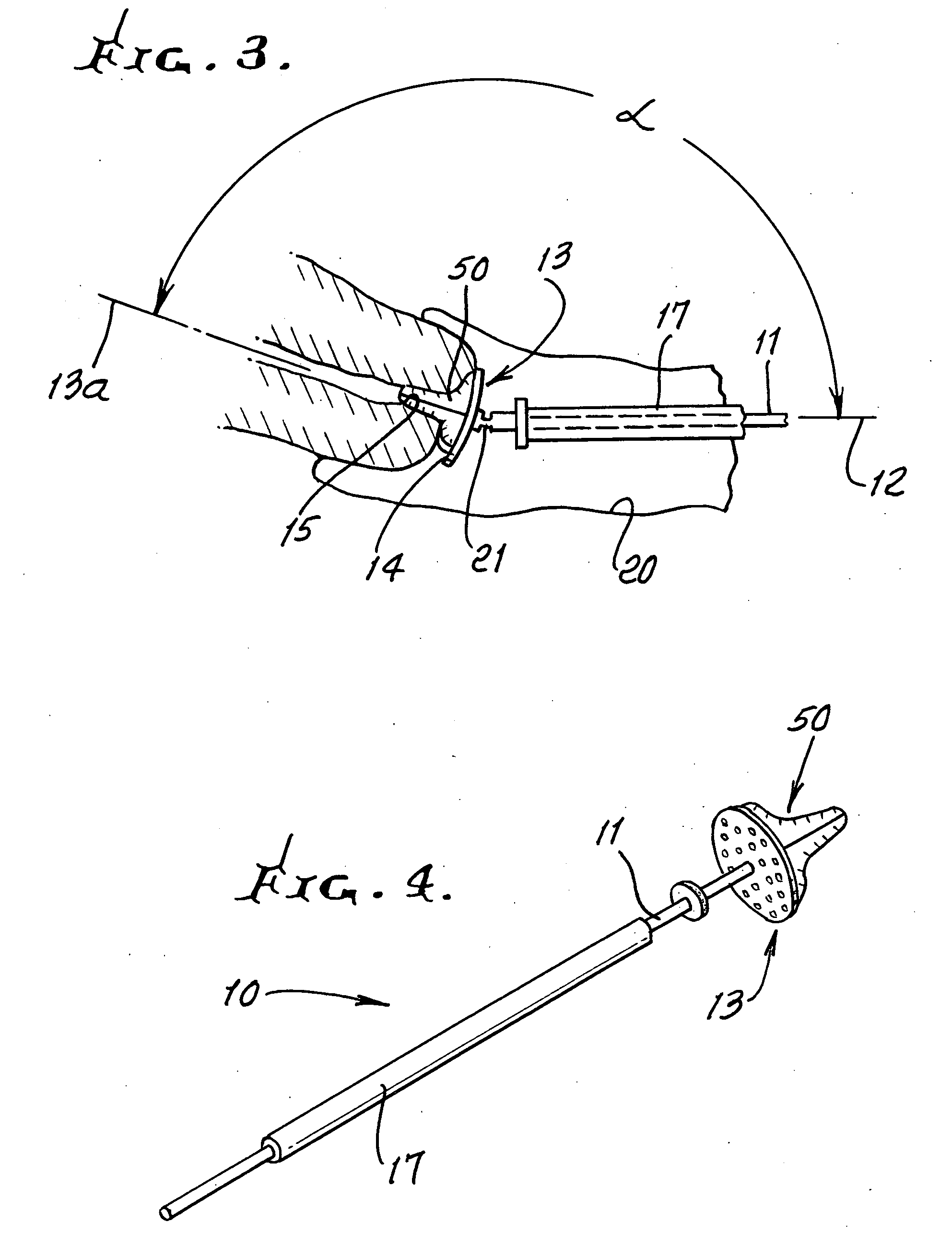
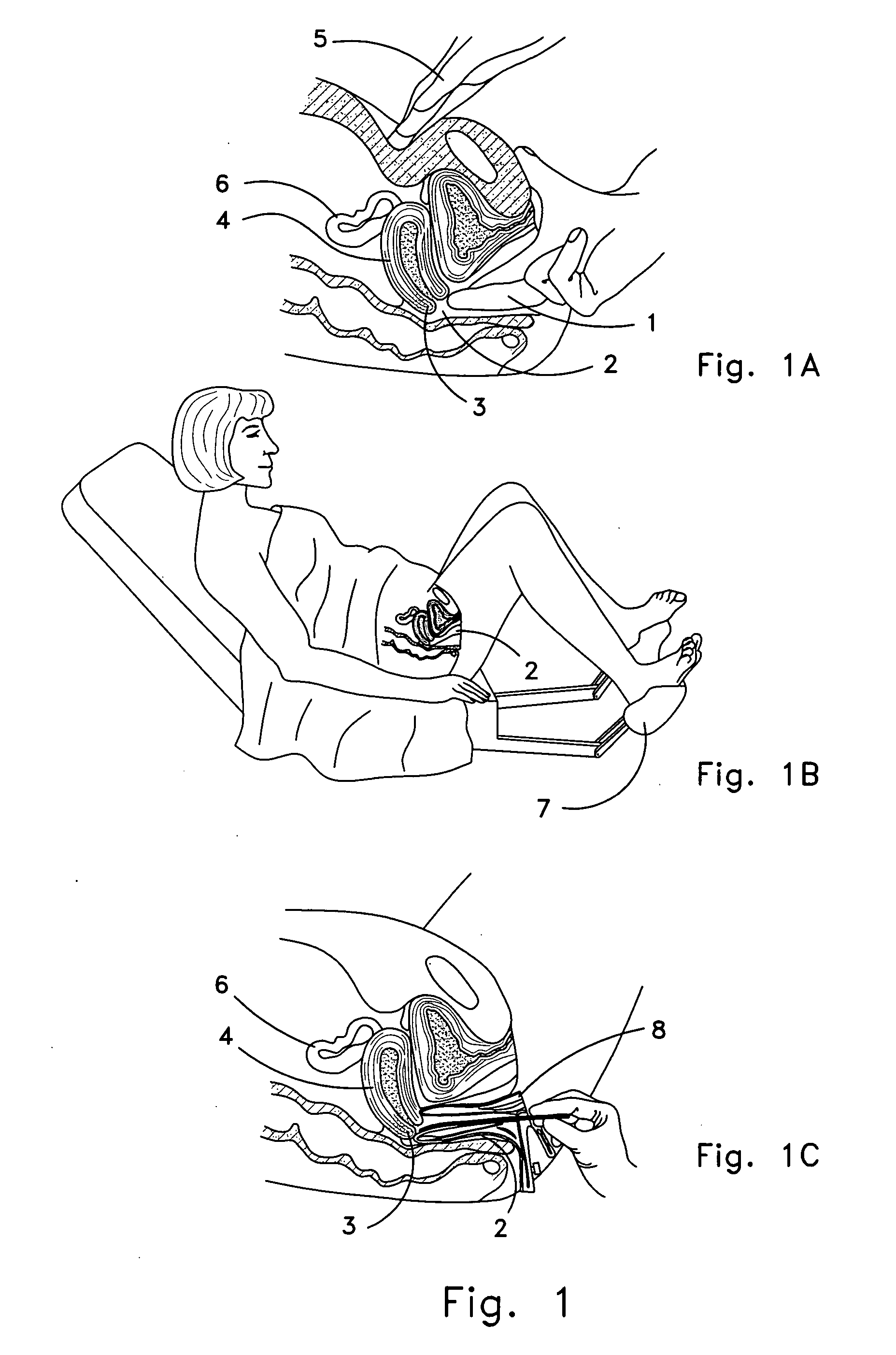
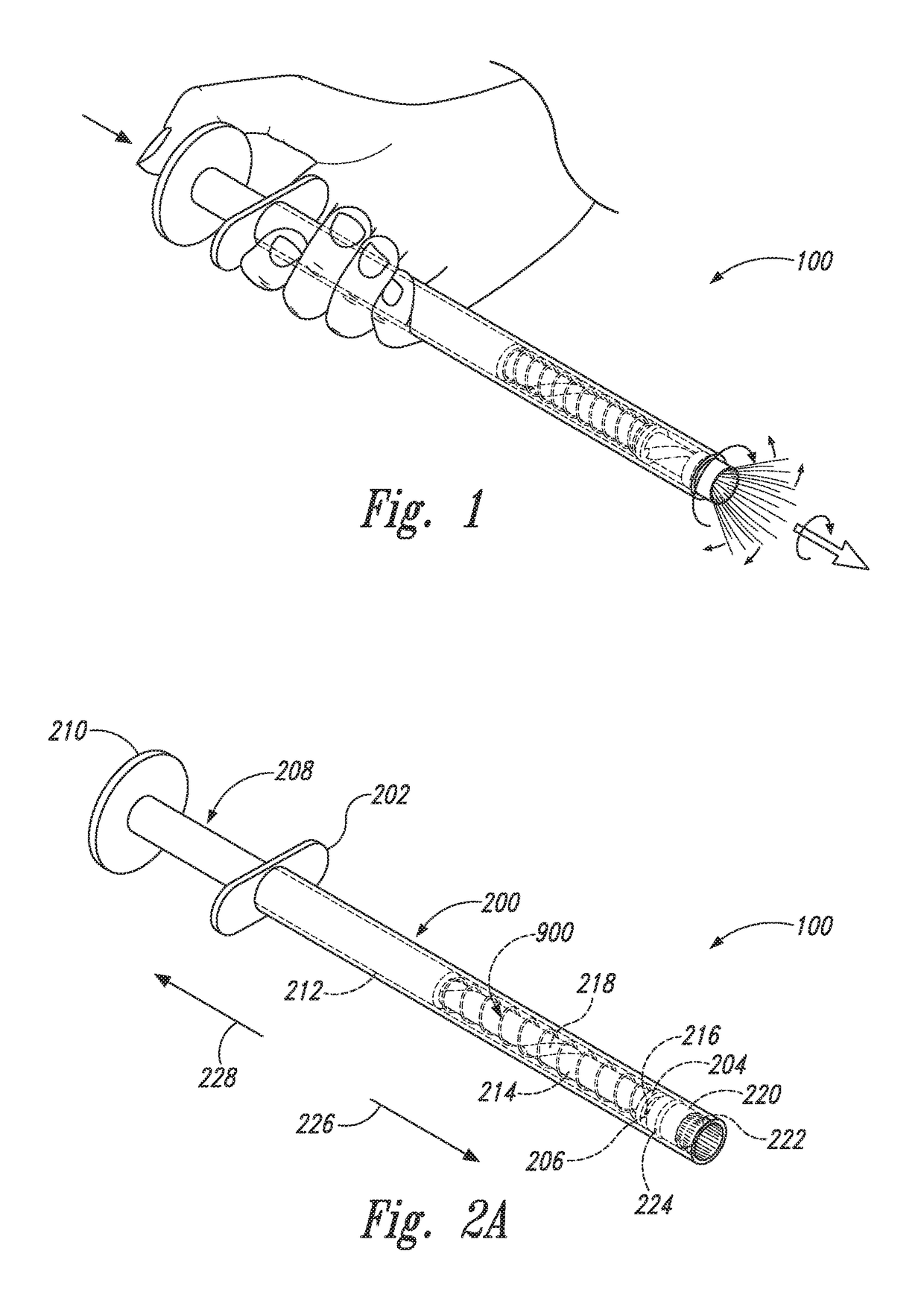
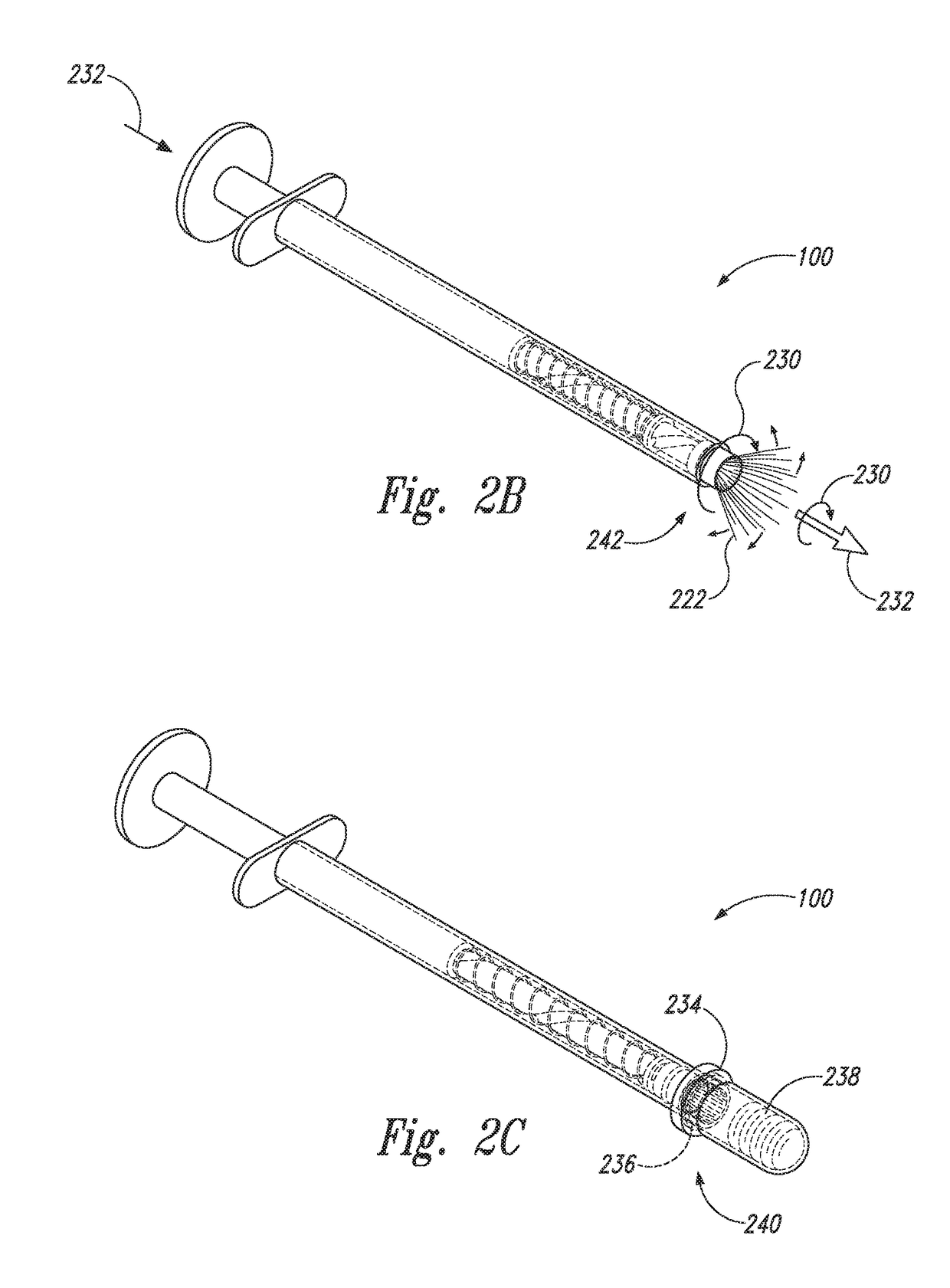
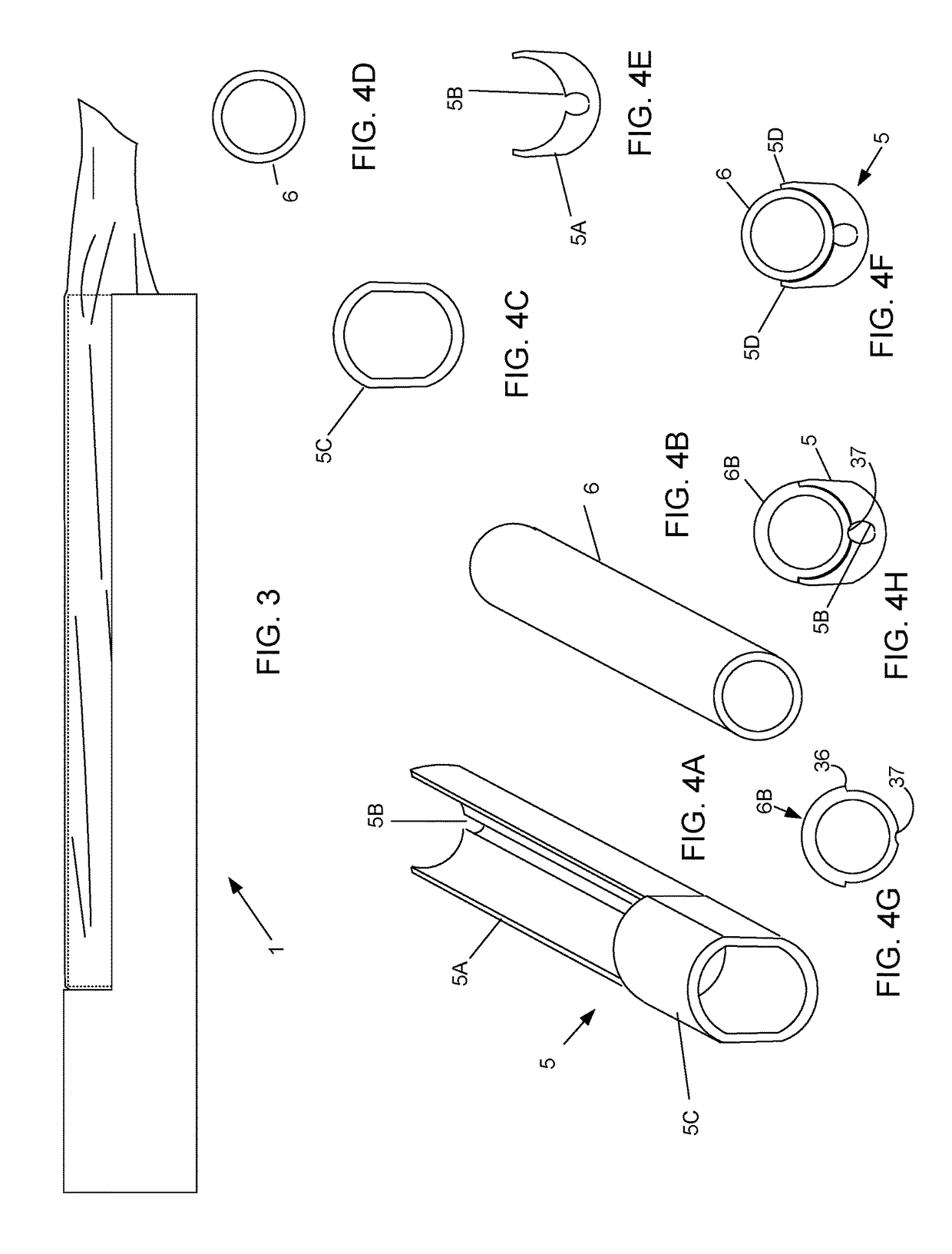
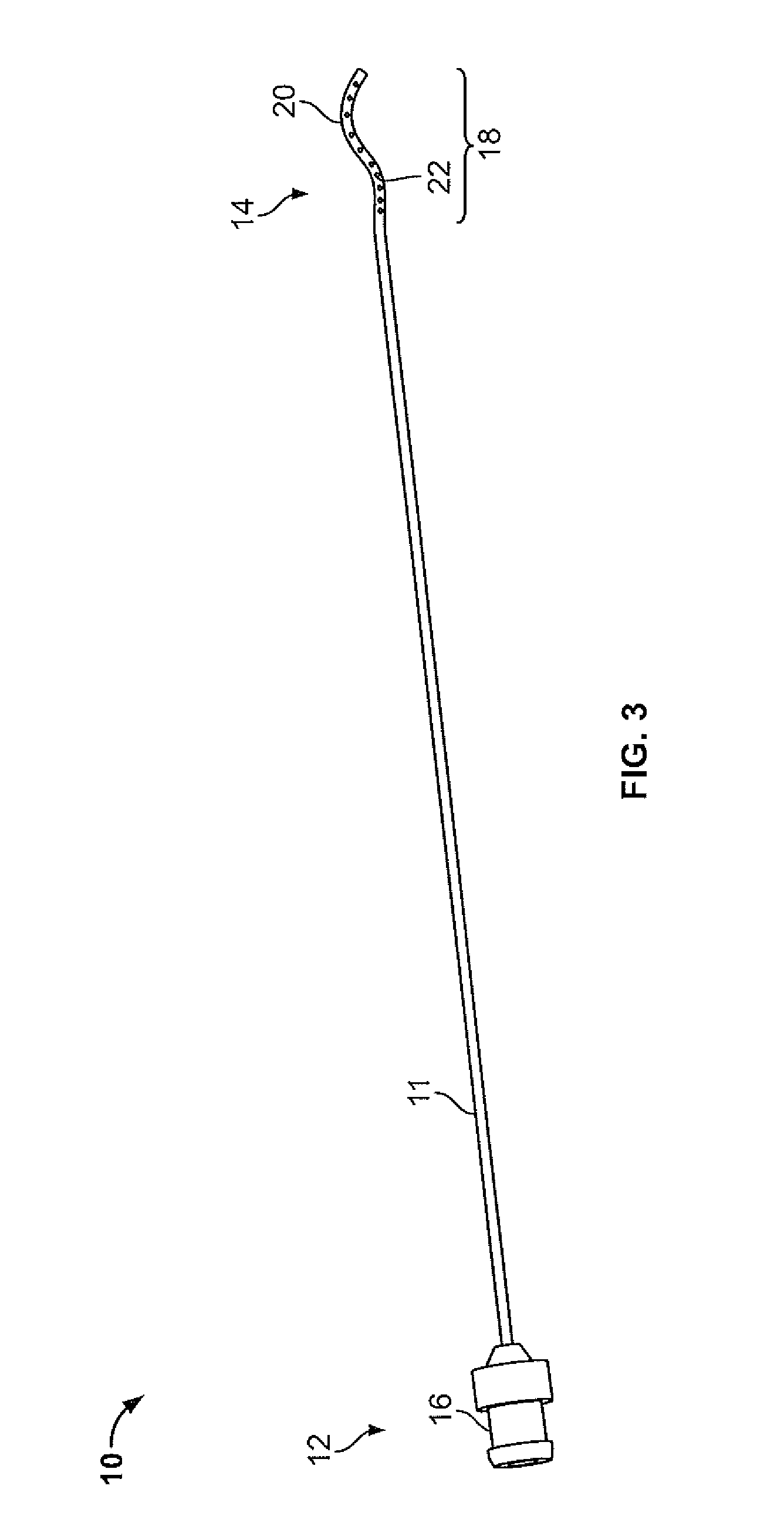
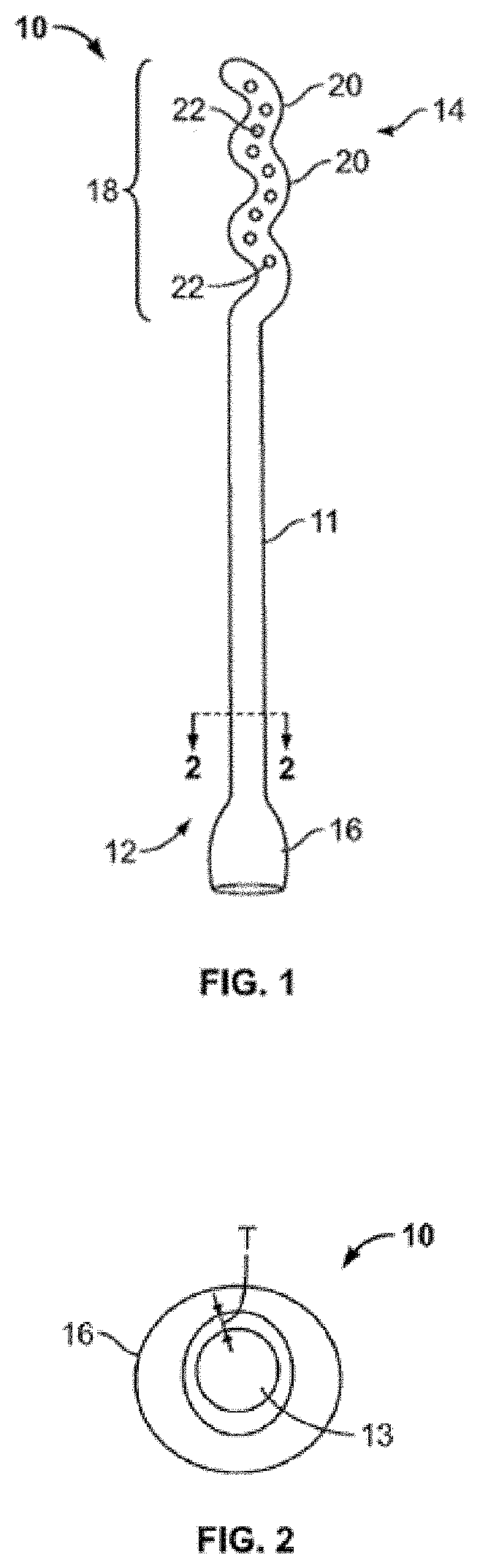
Device and method for conducting a pap smear test
A protective guide for protecting a cell extraction device during a Pap smear. A camera protective cover encloses a camera and a light emitting device. A thin, flexible transparent sheath encloses the camera cover. A cell extraction device cover encloses the cell extraction device. The location of the cervix is determined by utilizing the camera. The cell extraction device is pushed outward from the cell extraction device cover so that said cell extraction device head contacts the cervix and removes cells from the cervix. The cell extraction device is pulled back into the cell extraction device cover after the cells have been removed from the cervix. In a preferred embodiment the camera and light emitting device is an endoscope and the camera cover is an endoscope cover.
Owner:MILLARD MATTHEW D +2
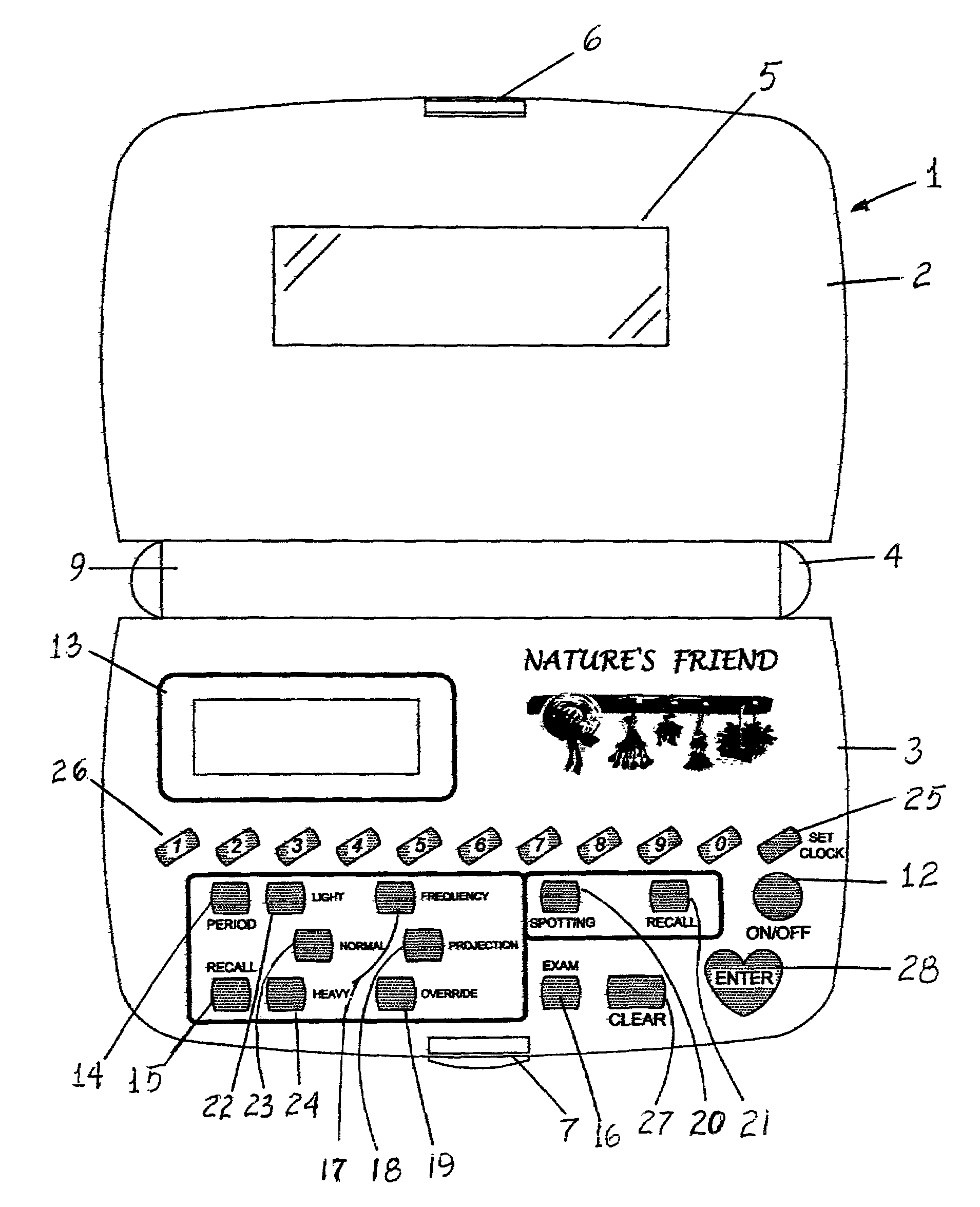
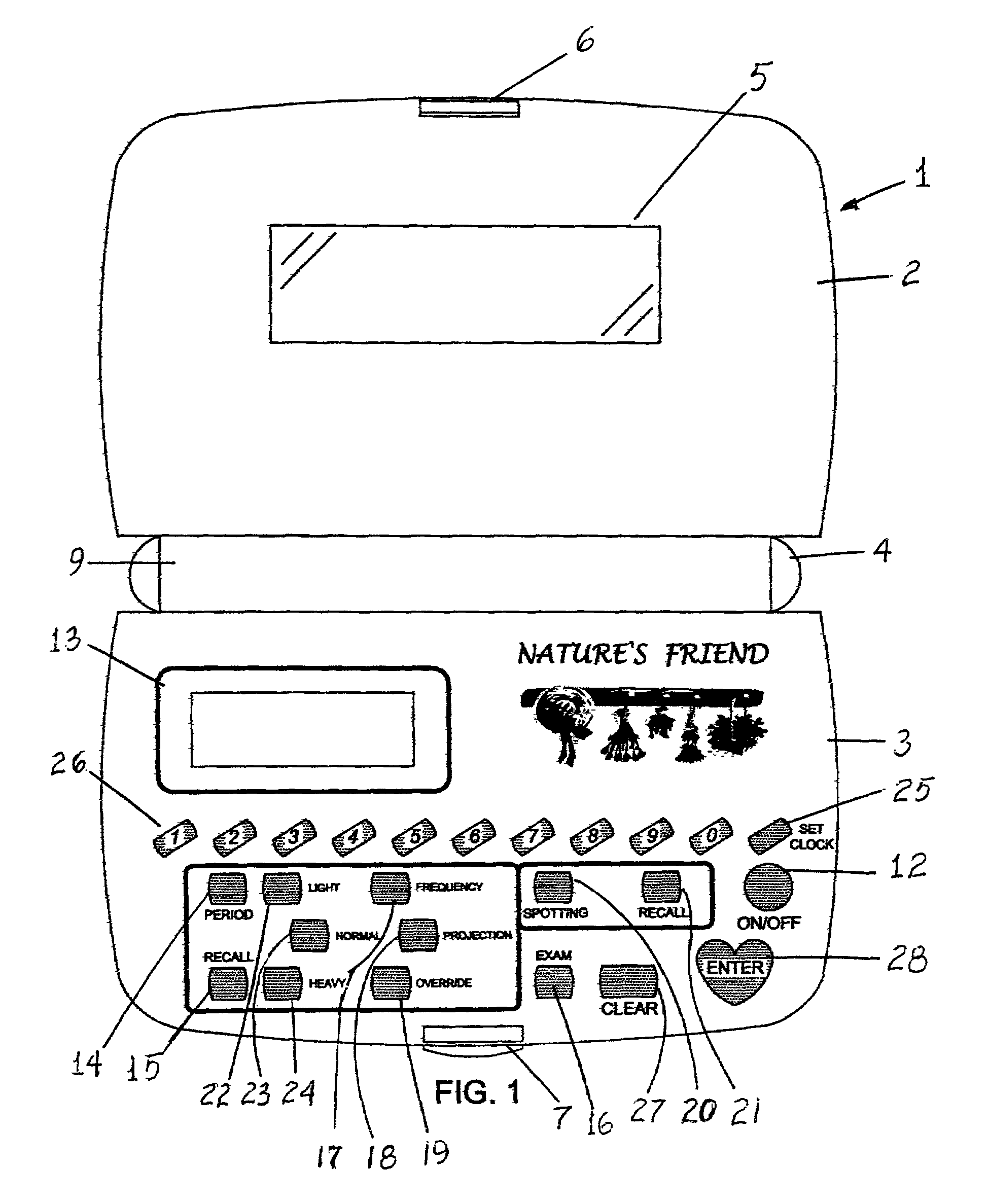
Nature's friend
InactiveUS6950839B1Simplistic in operationSimplistic in structureDigital data processing detailsDigital computer detailsGynecologyMenstrual cycle
An electronic device for recording a woman's menstrual cycle, recalling each recorded cycle and updating each cycle with respect to normal and abnormal menstrual flow; a frequency button is provided to record the average number of days between periods; a projection button is provided to project future period dates and is used in combination with an override button which recalls the number of days between periods and is usable to customize future period projection dates to an individual user's natural health response by setting and entering a new override value; an exam button is included to set and store breast and Papanicolaou, cervicovaginal or PAP smear exam dates and a reminder message is displayed for five seconds when the device is turned on once the stored exam dates are within two months of termination.
Owner:GREEN ALESANDRA +1
Pap smear sampling device and system
InactiveUS20090156962A1Compact storageEfficient use ofBioreactor/fermenter combinationsBiological substance pretreatmentsBristleEngineering
Owner:YONG PETER A K
PAP smear sampling device and system
InactiveUS7767448B2Efficient use ofProduced economicallyBioreactor/fermenter combinationsBiological substance pretreatmentsBristleEngineering
Owner:YONG PETER A K
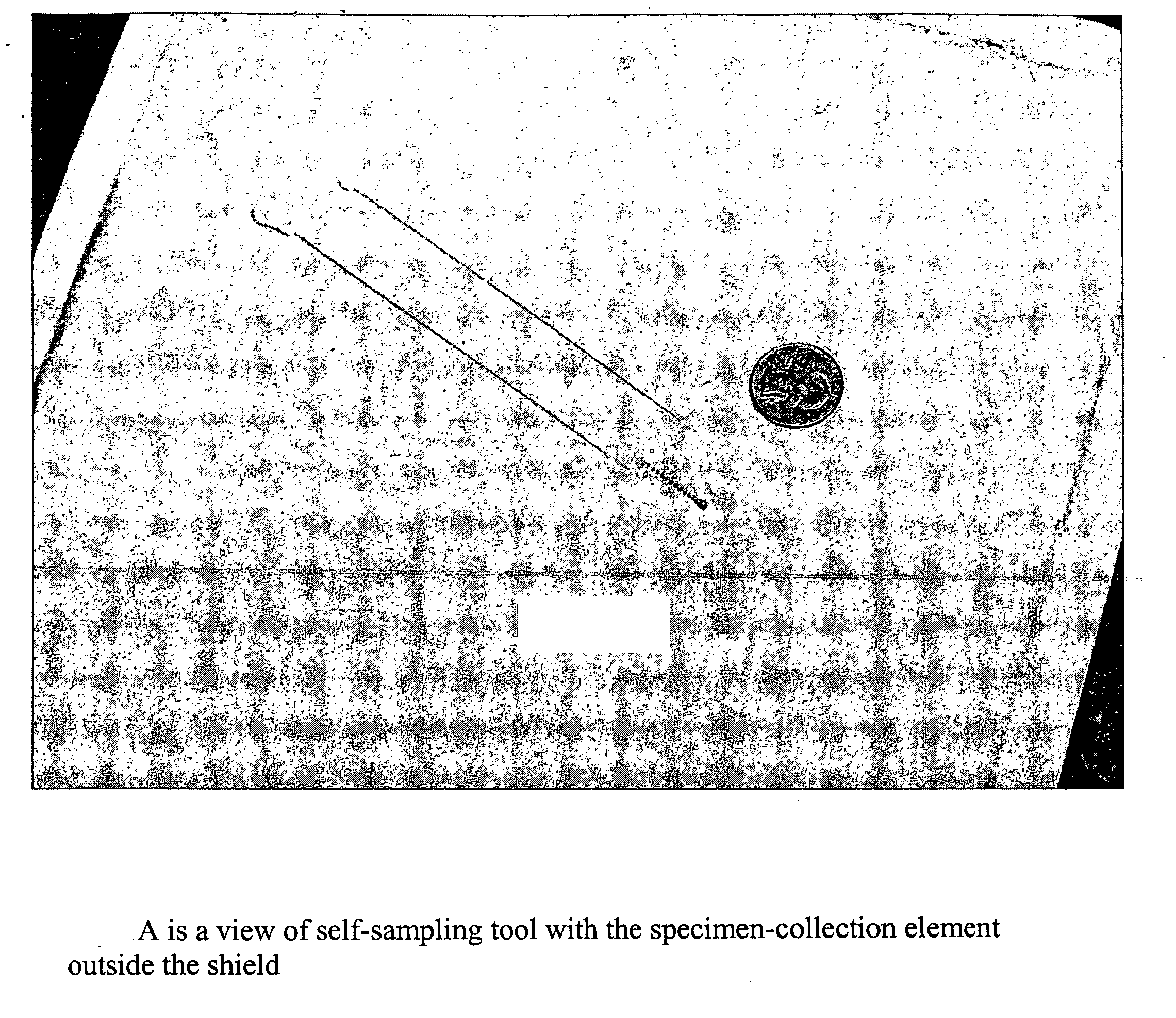
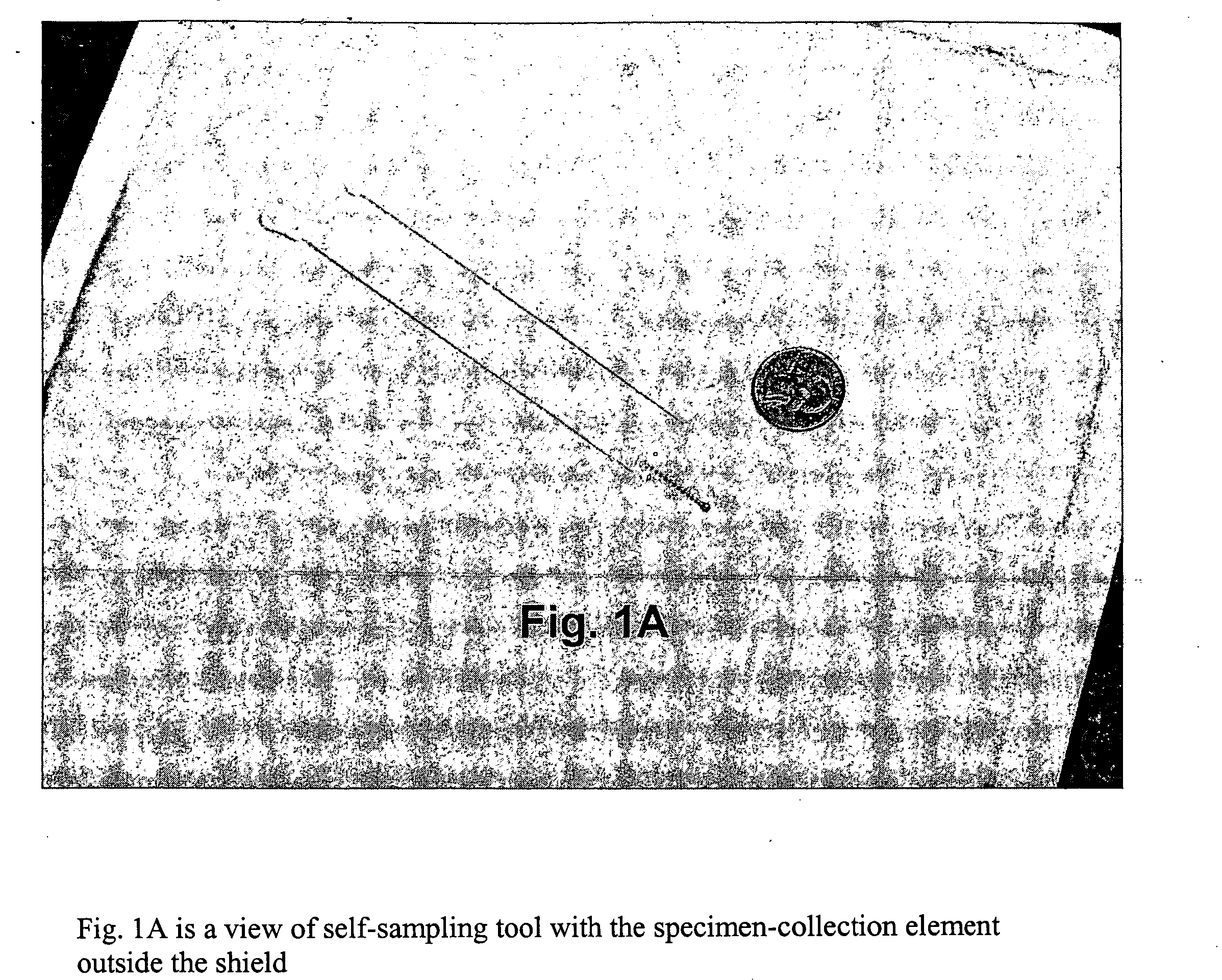
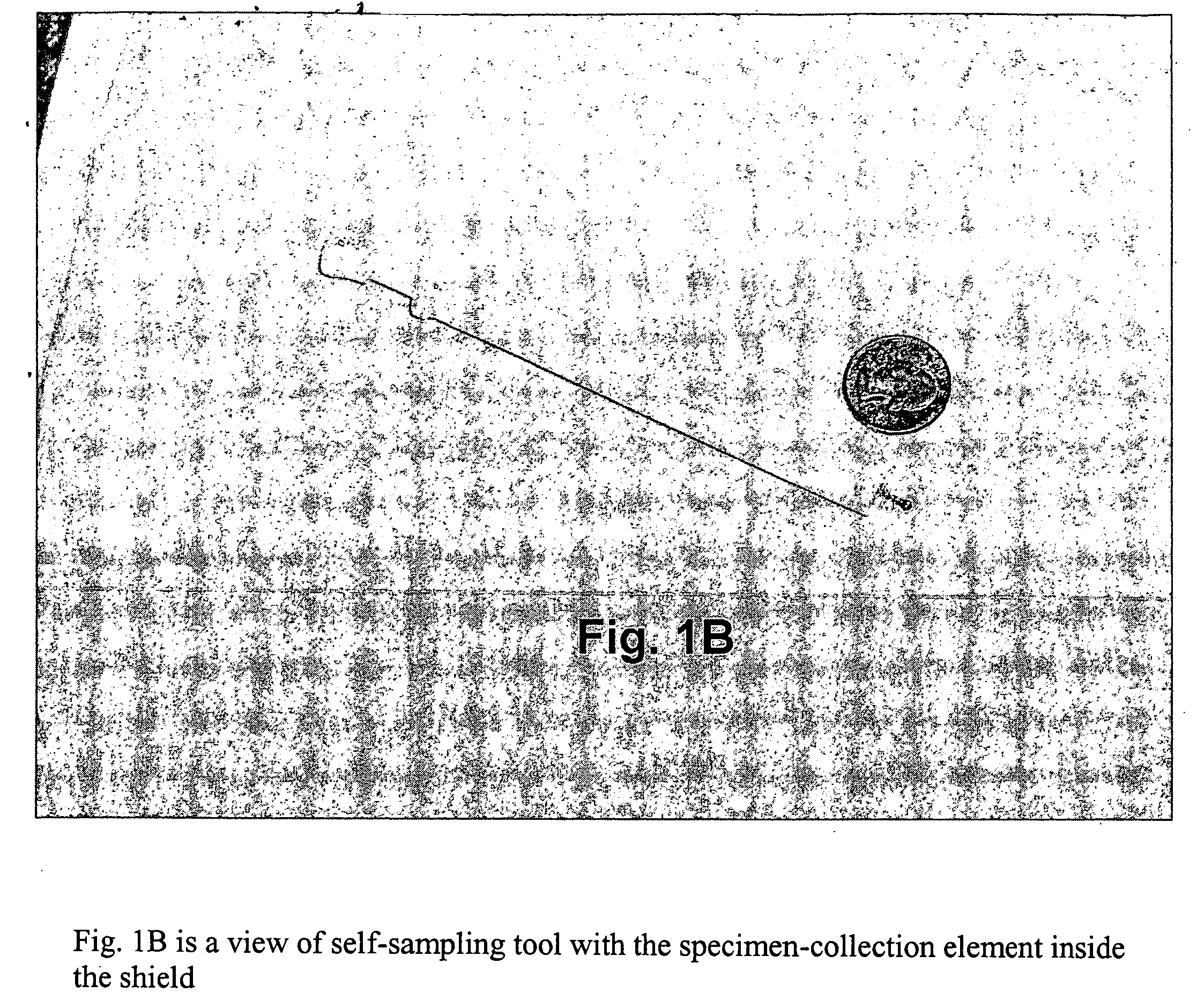
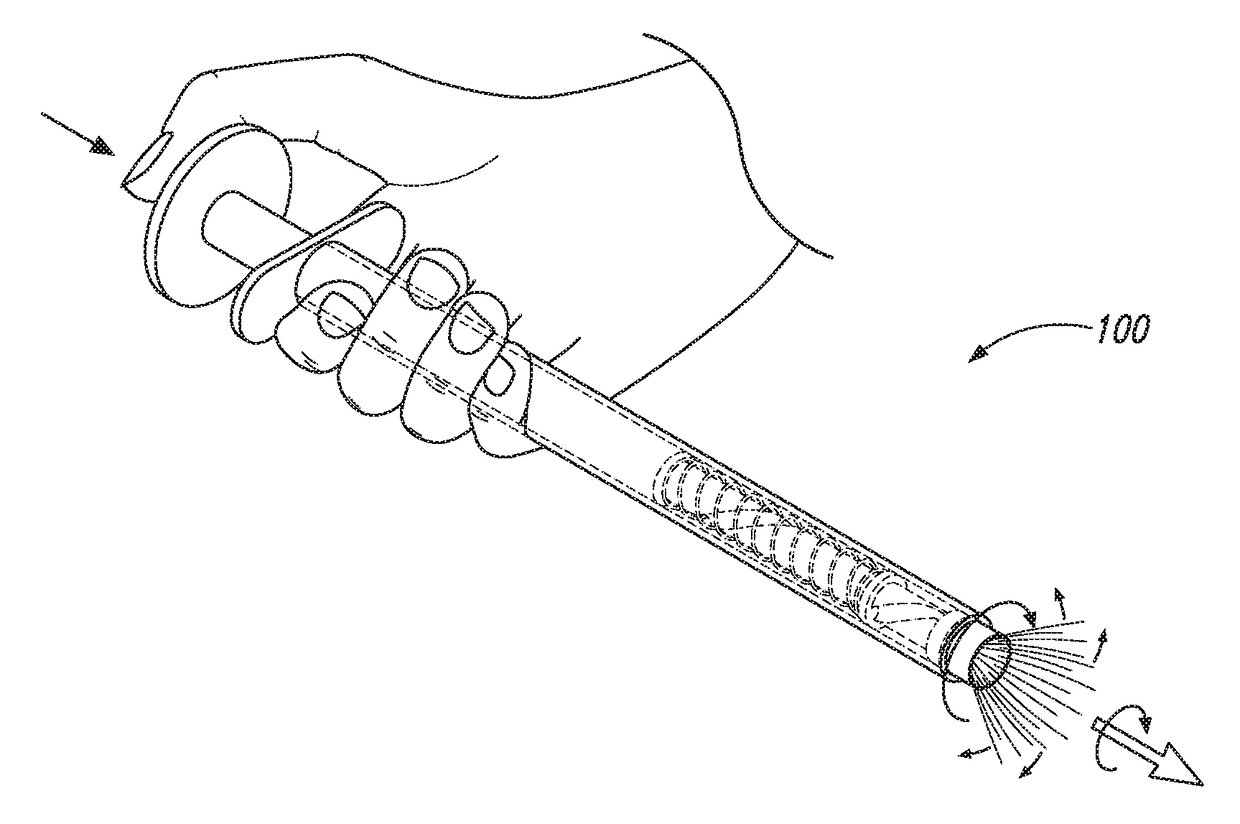
Cervical Cell Tissue Self-Sampling Device
ActiveUS20130066233A1Effective toolHigh densitySurgical needlesVaccination/ovulation diagnosticsCell adhesionSelf sampling
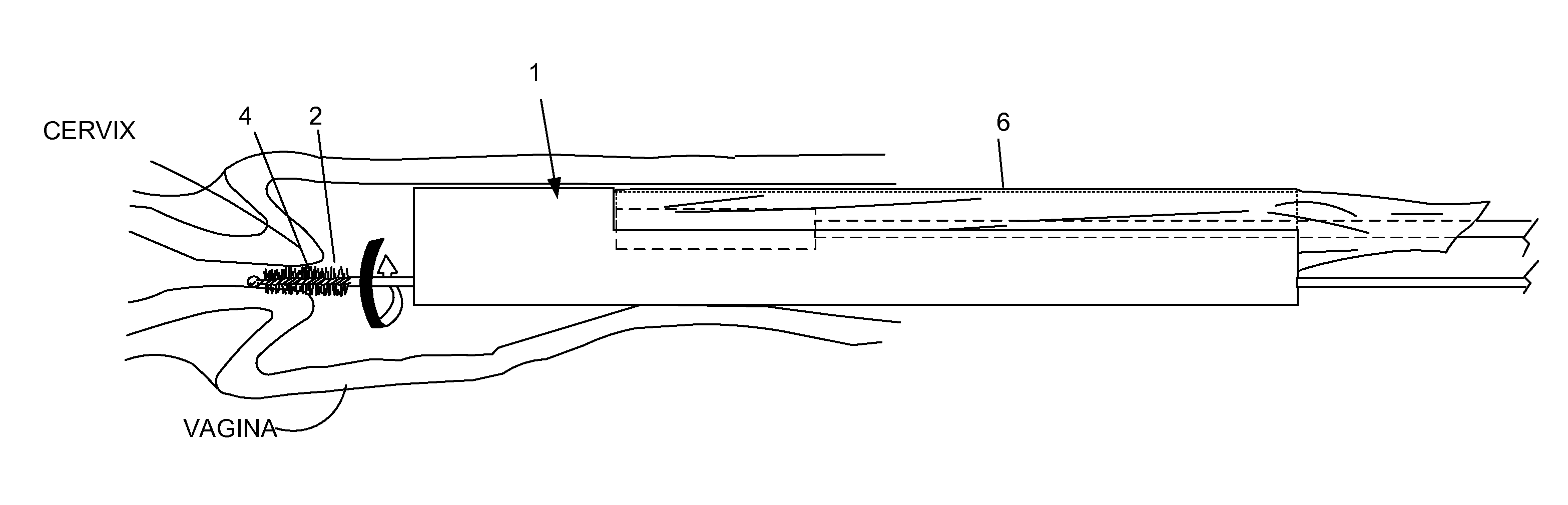
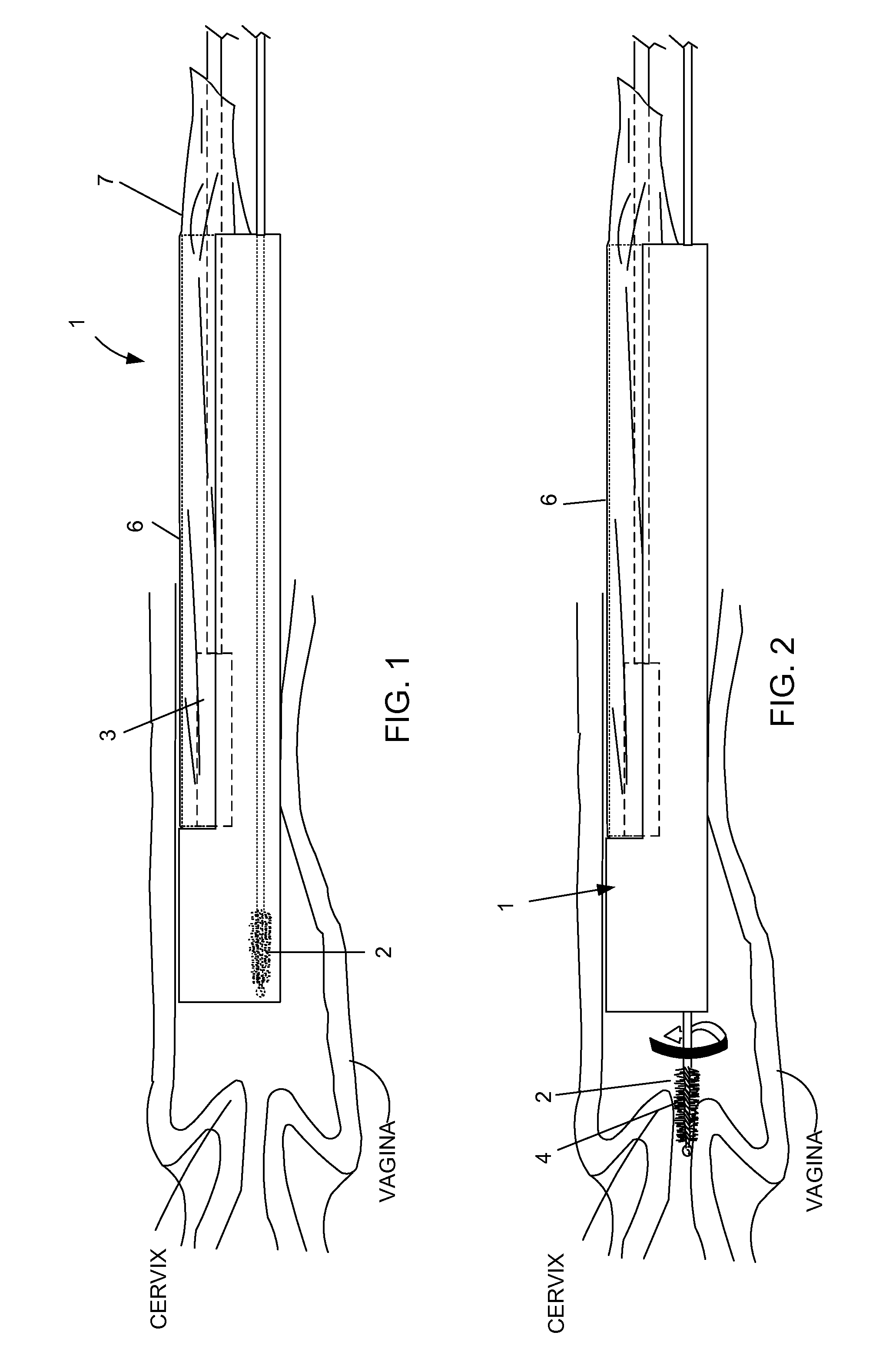
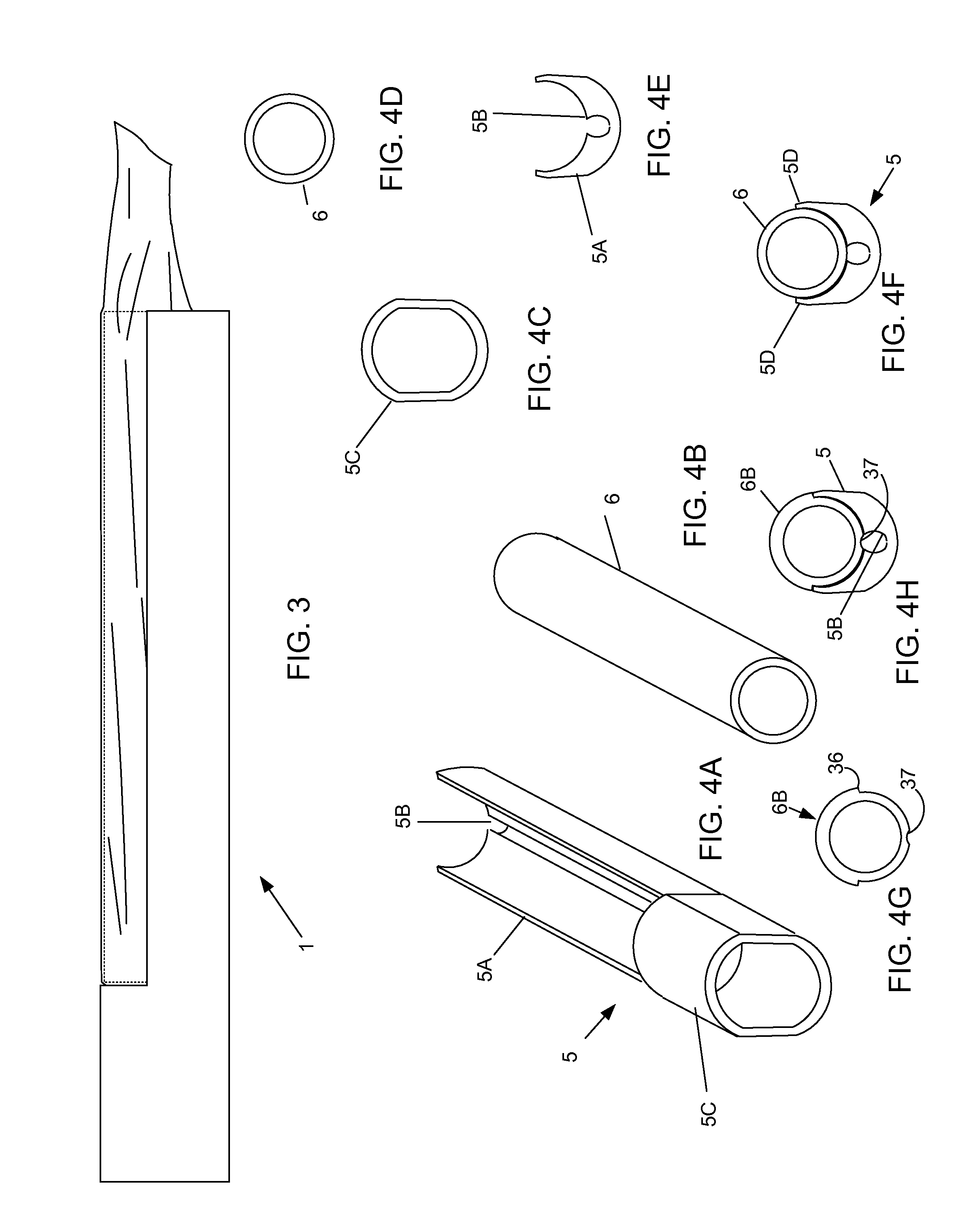
A device, a kit, and a method of use thereof, for self-administration and collection of cervical cell tissue samples such as for Pap smear testing. The device comprises an insertion tube, within which is carried a movable cervical aligning tool with an aligning probe, and a cellular sampling tool with a cellular adhesion surface. The aligning probe and cellular adhesion surface can be selectively movable relative to the insertion tube to improve accuracy of the testing and user safety.
Owner:GYNETECH
Cervical cell tissue self-sampling device
ActiveUS8460209B2Effective toolHigh densitySurgeryVaccination/ovulation diagnosticsSelf samplingGynecology
A device, a kit, and a method of use thereof, for self-administration and collection of cervical cell tissue samples such as for Pap smear testing. The device comprises an insertion tube, within which is carried a movable cervical aligning tool with an aligning probe, and a cellular sampling tool with a cellular adhesion surface. The aligning probe and cellular adhesion surface can be selectively movable relative to the insertion tube to improve accuracy of the testing and user safety.
Owner:GYNETECH
Pap smear collection device with ejection sleeve
InactiveUS20060200043A1Lower potentialControl releaseSurgeryVaccination/ovulation diagnosticsPregnancy testsPregnancy test
Sample collection for the Pap smear sample is critical for accurate diagnosis. Improper sample collection, poor sampling, and / or cell preservation can render a Pap smear unsatisfactory for evaluation, requiring a repeat smear collection. If the Pap smear does not contain appropriate representative cells from the transformation zone and endocervical canal, the ability of the test to detect disease is very low. Likewise, if the preservation of the sample is compromised, the screener's ability to recognize abnormal cells is greatly diminished. It is generally understood that cervical samples should be harvested by a two-stage technique, which includes sampling of the endocervical canal with a cytobrush and obtaining a sample from the transformation zone with a spatula. The use of either the cytobrush or the spatula alone may be adequate but not as effective as the two-stage technique. Both the Cervex Brush and REG;(Unimar, Inc.) and the Accellon Combi & REG;(Medscand AB) are two collection devices which combine the action of the cytobrush and spatula, thus permitting broader sampling with a one stage technique. The standard method of transferring cervical cells from the collection device or complete transfer of the collection device into the liquid collection vial is often challenging. These challenges include but are not limited to: 1-spilling the sample, 2-dispersing the medium collection such that air born body secretions could contact unprotected health care workers, 3-missing the collection container and contaminating the sample. It is the goal of the current device embodiment to provide a simple and consistent method of transferring the entire collection specimen into the collection container to maximize cell collection while minimizing challenges of head disengagement. Such devices are not limited to the cervical cell cytology collection markets but extend to all cell collection methods were the entire sample is suspended in a liquid or similar medium. Such samples include oral cavity collections (throat swabs, vaginal cavity collections (STDs, pregnancy test), urethral cavity collections (male STDs) fornex collections (Alaph fetal protein), and rectal collections as well as open procedures requiring cell sampling.
Owner:JANNETTY JOSEPH D +1
Digital Cerviscopy Device and Applications
The present invention relates to a digital system for cervical disease screening and detection, with clinical application as adjunct to Pap smear. The device of the present invention is designed for insertion into a woman's genital cavity, and is composed of a digital camera and light source at one end to take pictures of the cervix and its environment, of a flexible tubing connecting said end to a distal end, and of a distal end comprising a digital mean with capacity to store images, display, transfer and share them as required, on-site or at a remote location. This instrument can either be used by a health professional, or self-operated.The present invention provides a system making cervical disease screening routinely accessible to every woman, by improving frequency and cost-effectiveness of cervical disease surveillance in general, and facilitating access to health care professionals even to women in rural or underserved areas.
Owner:MILAGEN
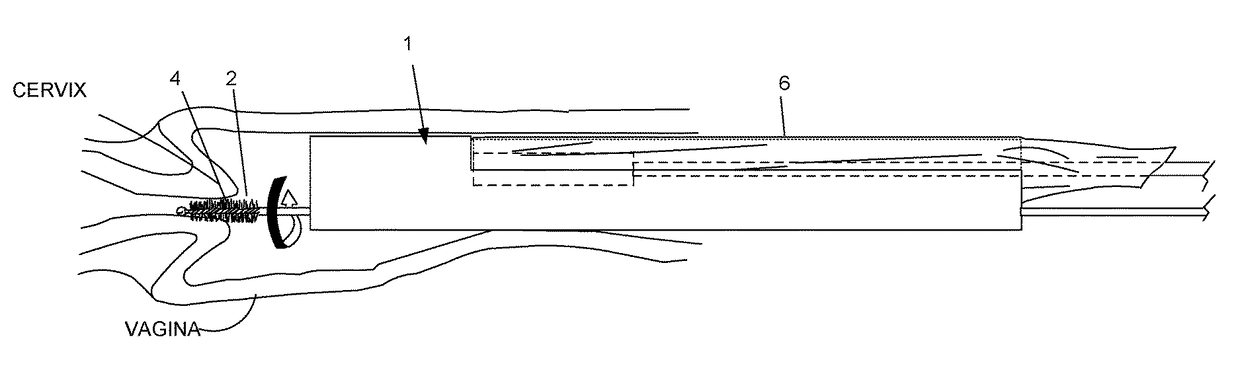
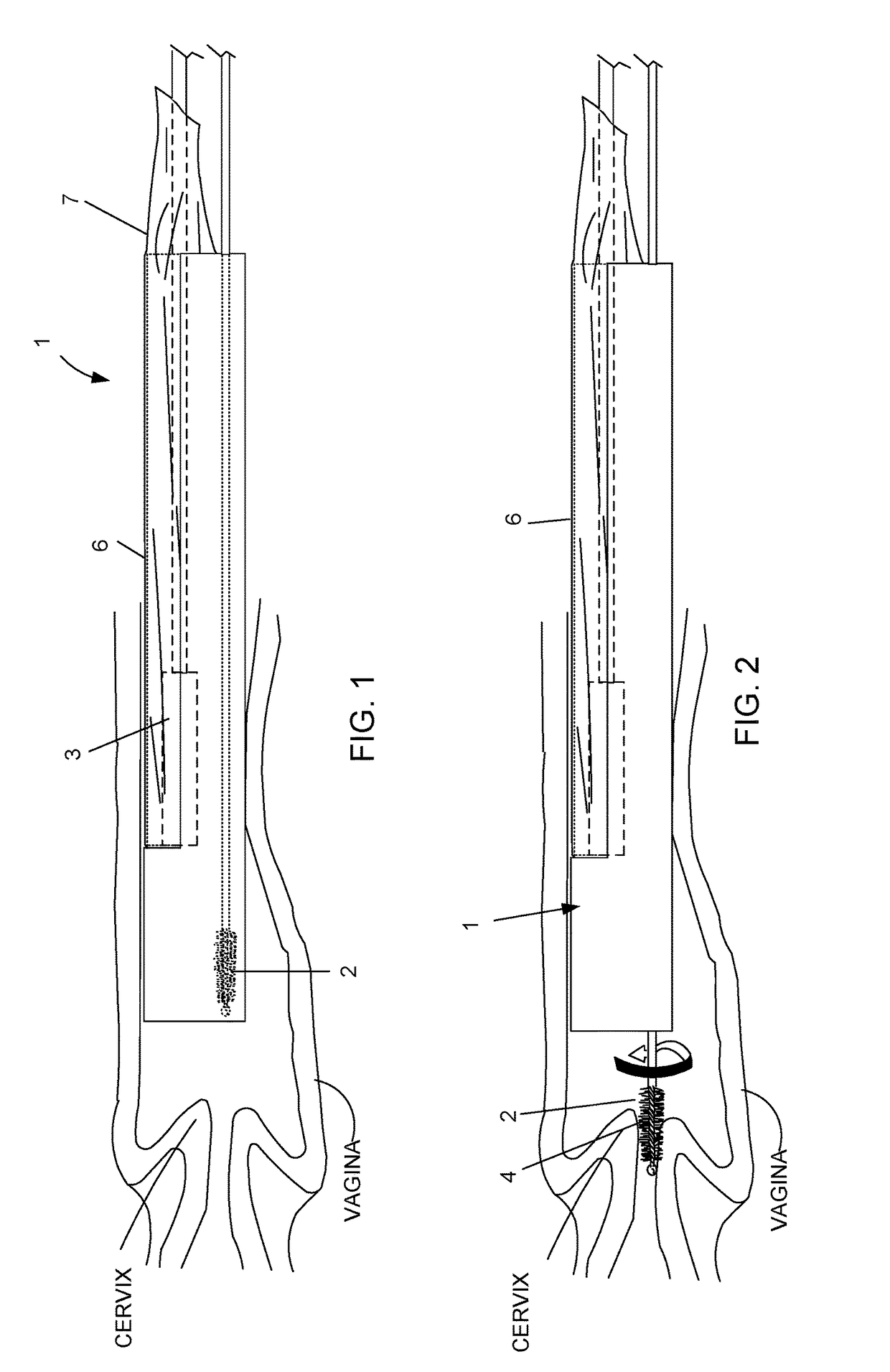
Mucous gathering for Pap smear testing
InactiveUS20070270713A1Increase contactEliminate areaSurgeryVaccination/ovulation diagnosticsProximateCervix
A mucous gathering device, comprising an elongated rotatable stem having an axis, and a rotary head piece carried at an end of the stem for flexing; the headpiece having a face sized for presentation to the cervix entrance as the head rotates about an axis angled relative to the stem; and the face having associated structure for gathering mucous proximate the cervix as the head rotates.
Owner:NG RAYMOND C
Apparatus and method of personal screening for cervical cancer conditions in vivo
InactiveUS20040068162A1Early cancer detectionData generationSurgeryVaccination/ovulation diagnosticsGynecologyMenstrual cycle
A method and apparatus for personal screening for early signs of cervical cancer is claimed, whereby the user performs daily or almost daily a diagnostic self-check for some other aspect of reproductive health, and the electronic testing for cervical cancer type of tissue aberration is performed automatically in the background. The screening is invisible to the user, causing no anxiety and no discomfort. The user only becomes alerted to the need to see a physician if a preset condition of reproducibility is reached in the background evaluation of the measurement data if the aberrant pattern has been detected consecutively in a preset number of menstrual cycles, the device prompts the woman to see a physician with a view to undergoing a more demanding definitive diagnostic examination such as colposcopy with biopsy. The invention provides the diagnostic screen in a manner that does not cause the discomfort, anguish and anxiety associated with the Pap smear screen of the prior art.
Owner:KIRSNER VACLAV
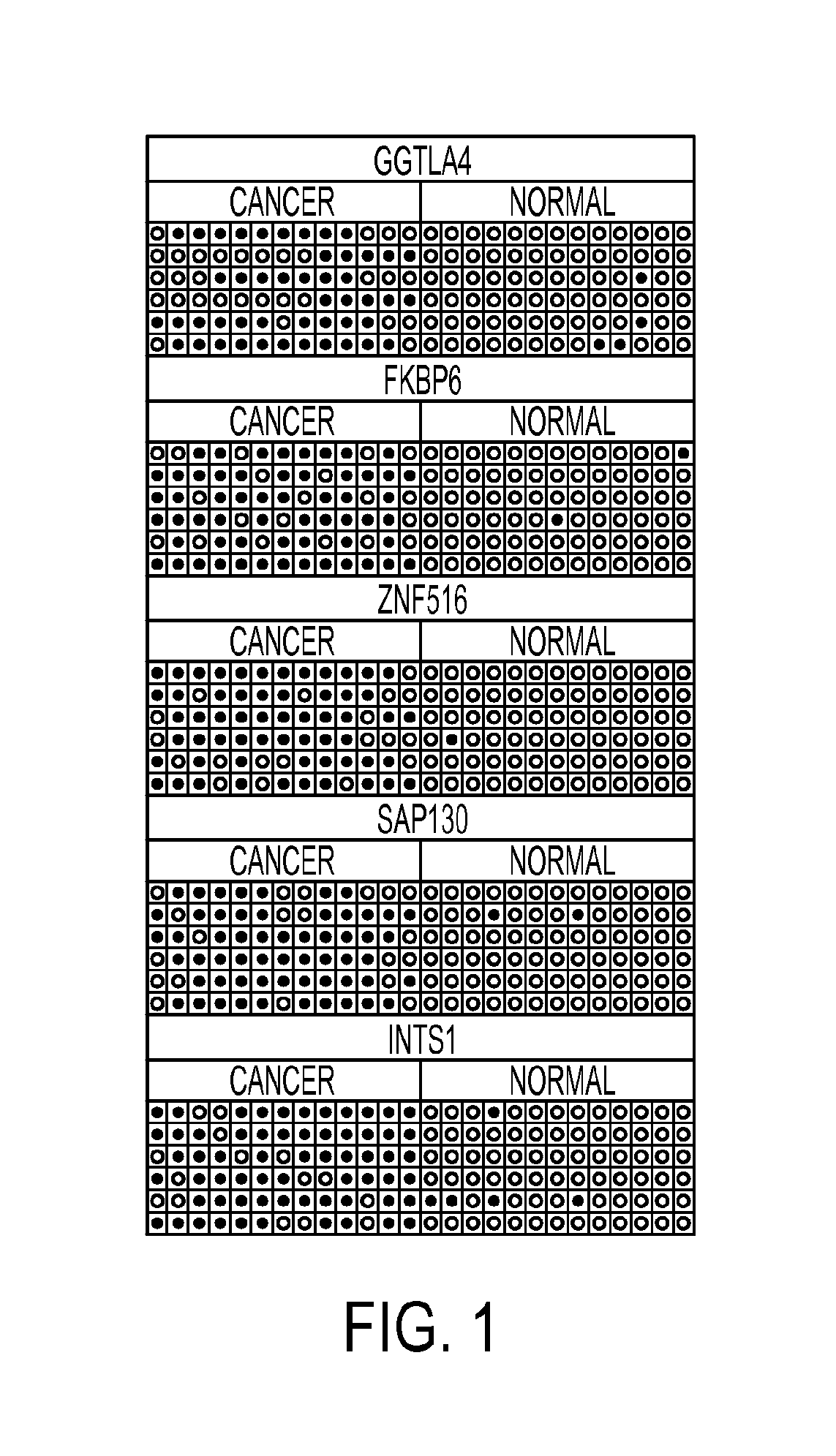
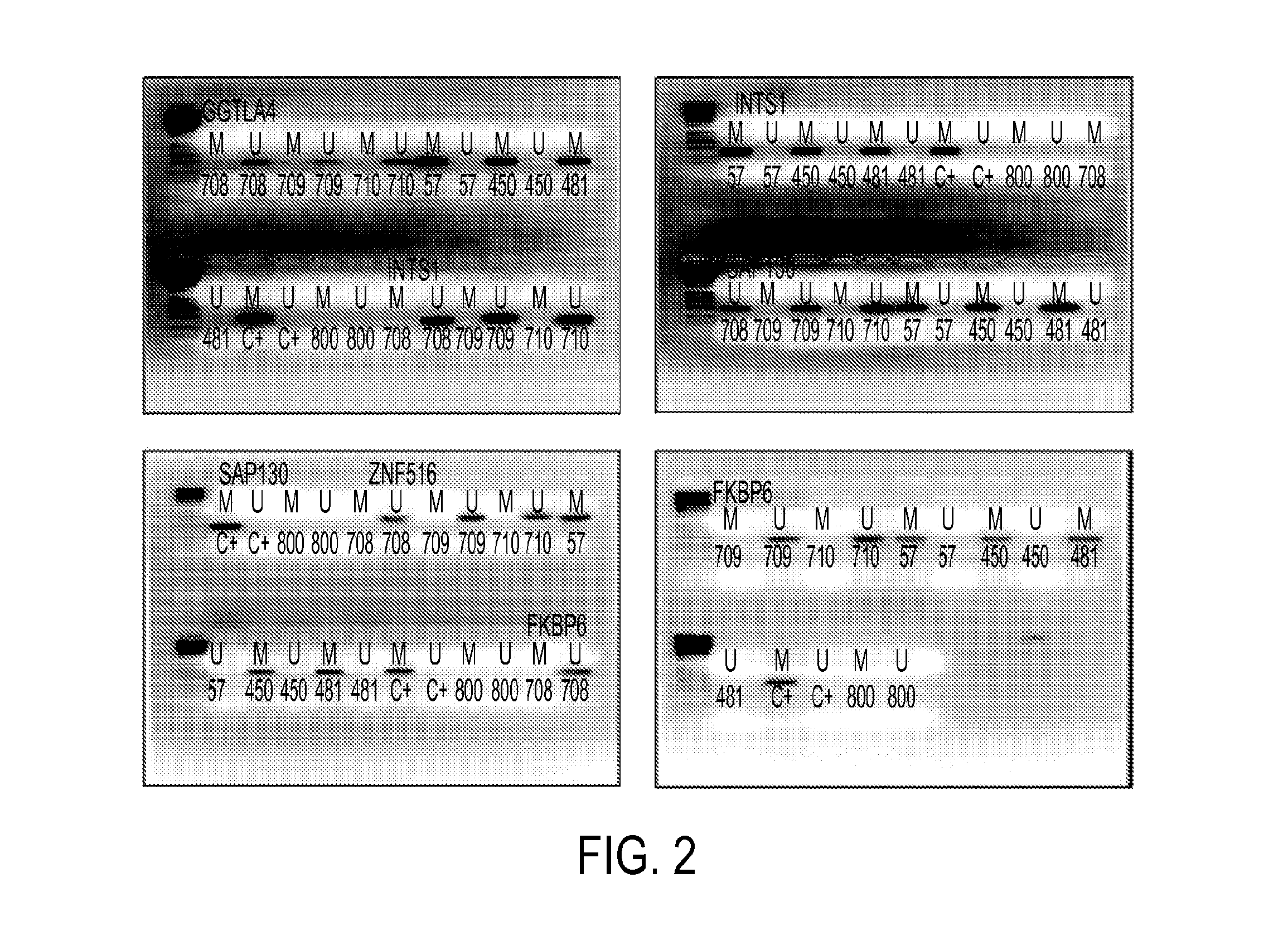
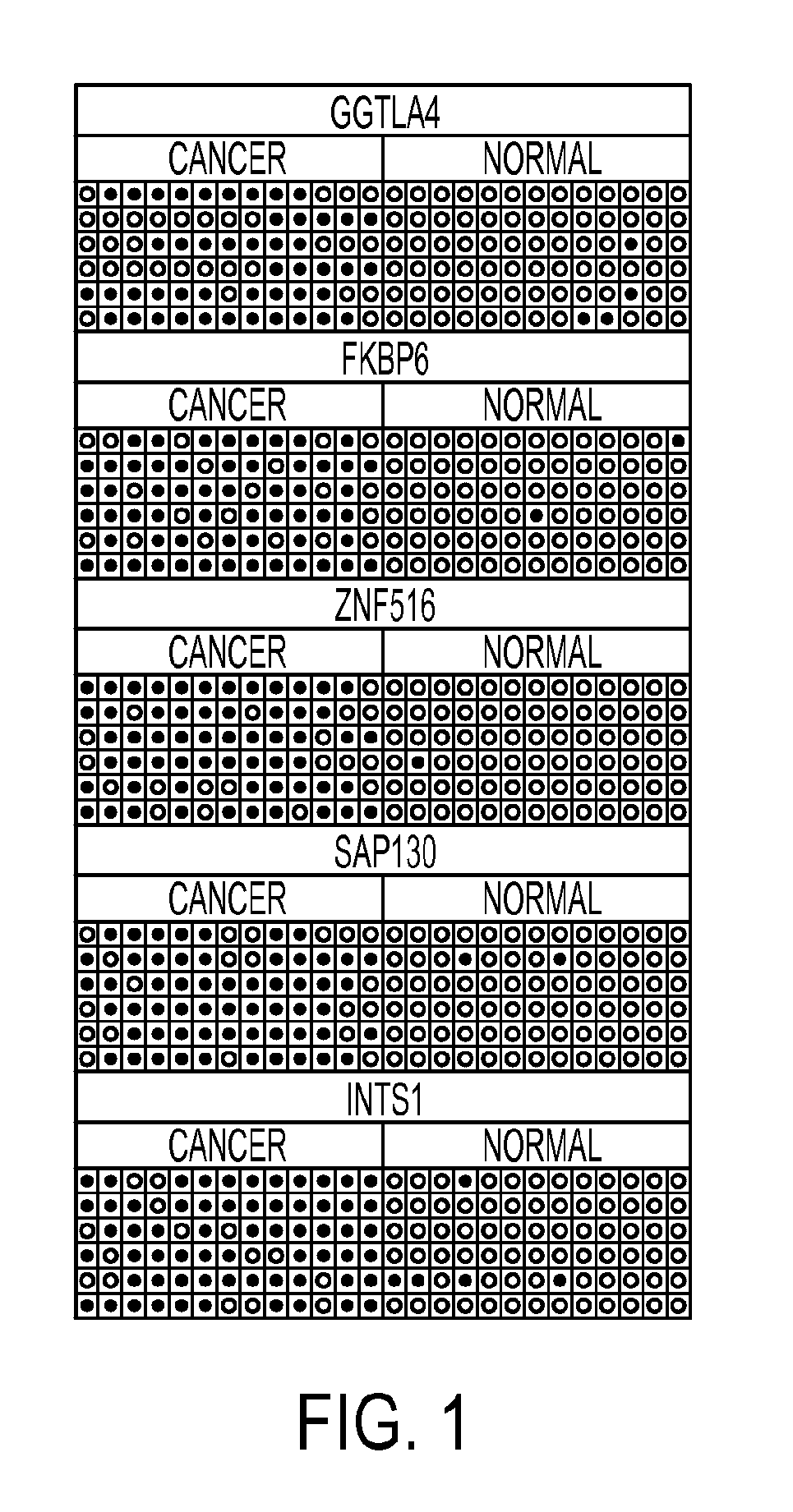
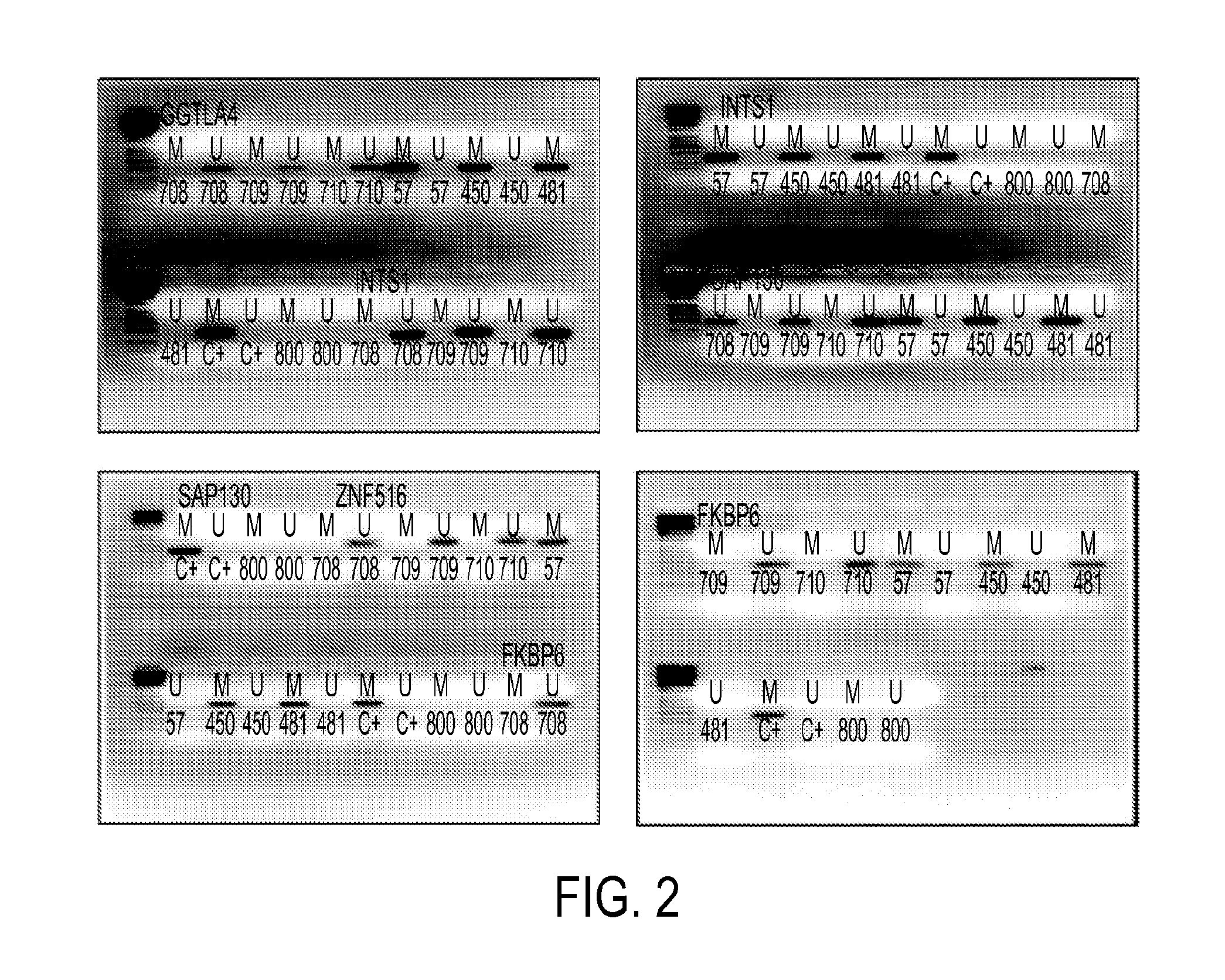
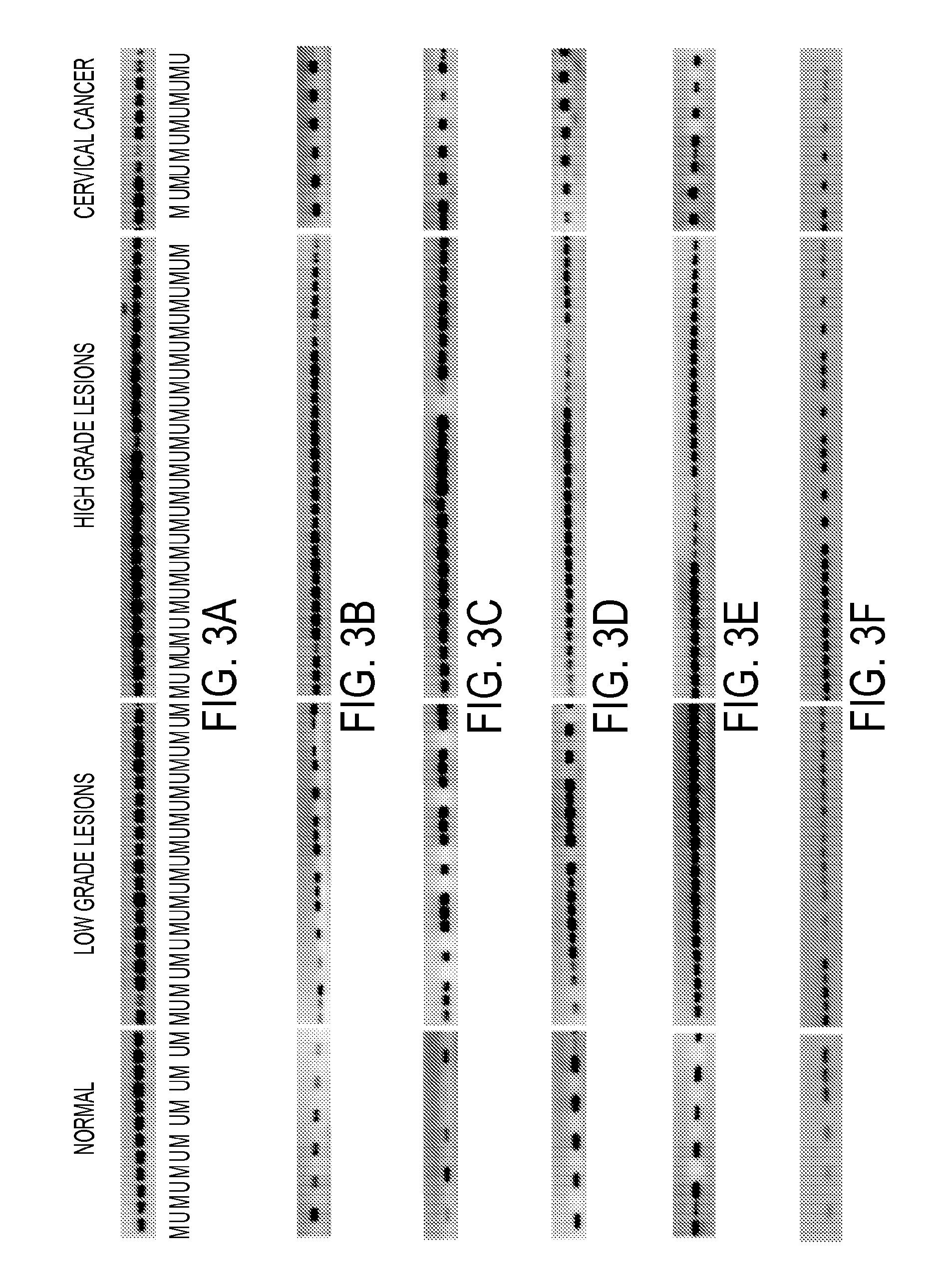
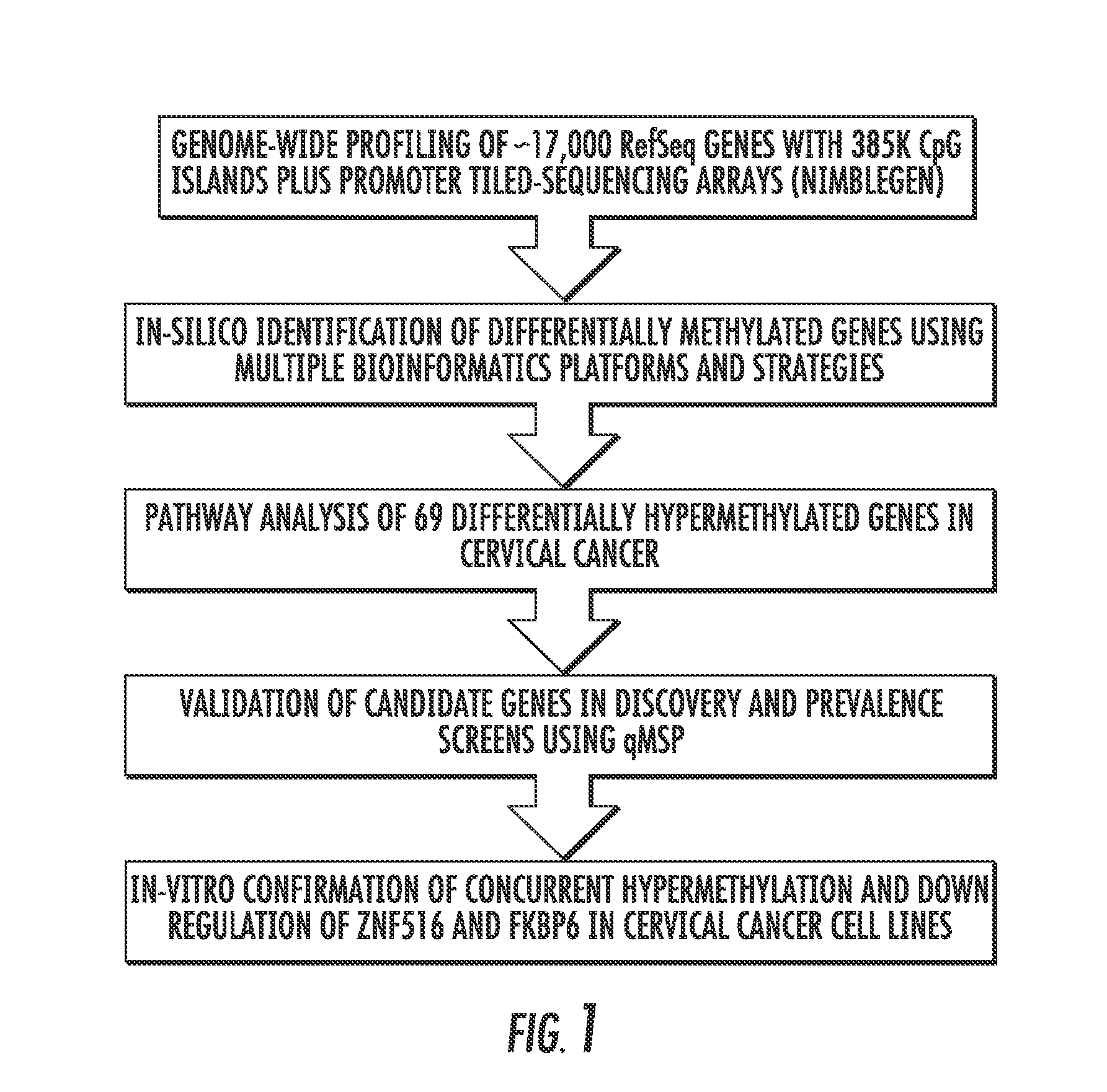
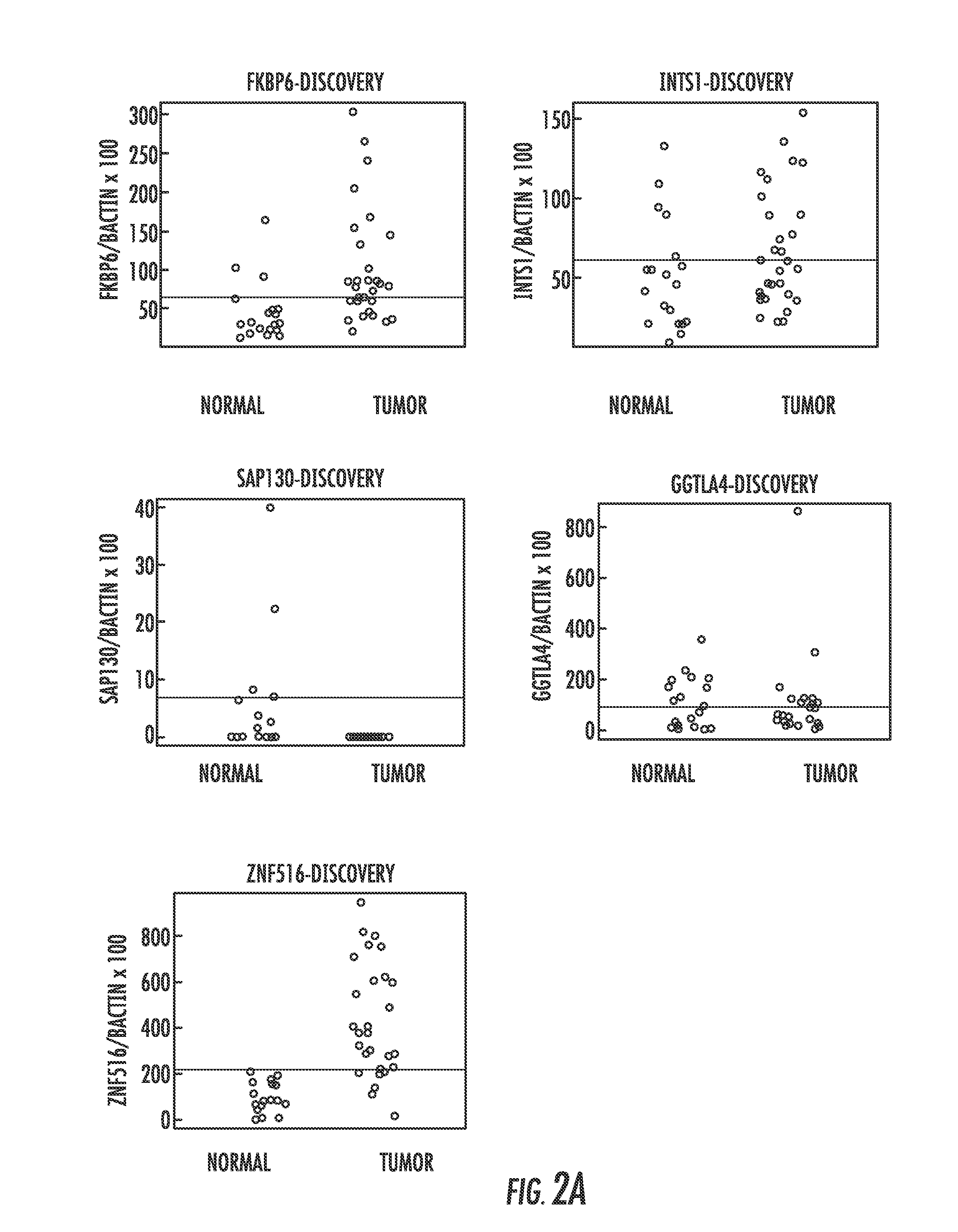
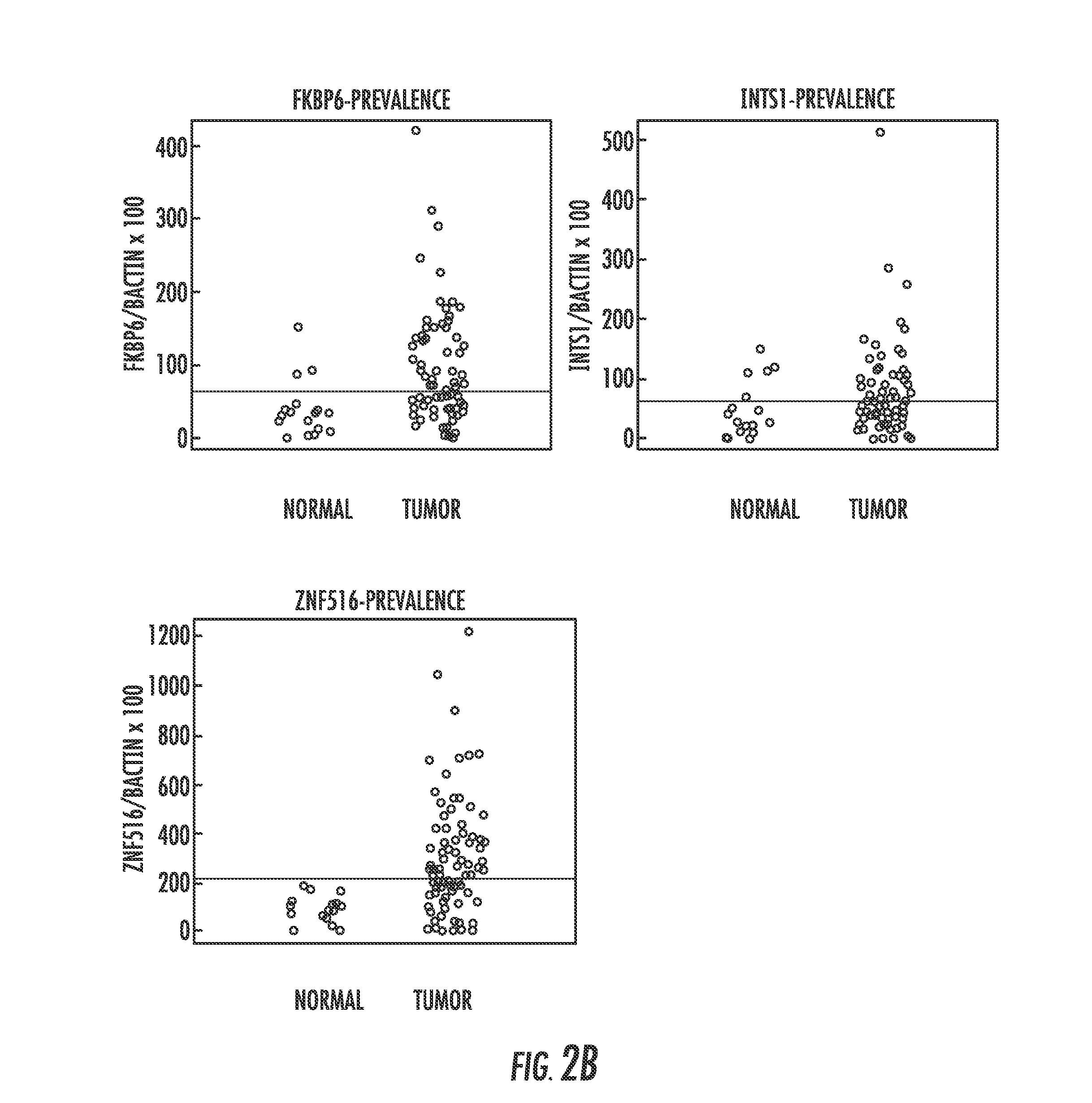
Hypermethylation biomarkers for detection of cervical cancer
ActiveUS8859468B2Nucleotide librariesMicrobiological testing/measurementPotential biomarkersOncology
Pap smears and HPV infection tests do not distinguish between lesions that will progress to an invasive carcinoma and those that will not. We aimed to identify epigenetic biomarkers for diagnosis and progression monitoring of premalignant lesions in cervical cancer. Hypermethylated genes were identified as potential biomarkers after validation by MSP, including GGTLA4 and ZNF516. The methylation frequency for these two genes was higher in tumor: GGTLA4 (100%) and ZNF516 (96%); than in normal samples: GGTLA4 (12%) and ZNF516 (16%). The methylation status of GGTLA4 showed a progression in methylation frequency from normal samples to invasive carcinoma. The immunohistochemical expression was lower in tumor for both: GGTLA4 (50.8%) and ZNF516 (66.2%); than in normal samples: GGTLA4 (71.2%) and ZNF516 (88.1%) (p<0.05). In conclusion, we identified methylation biomarkers for the molecular screening and characterization of cervical cancer.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Device and method for conducting a pap smear test
A protective guide for protecting a cell extraction device during a Pap smear. A camera protective cover encloses a camera and a light emitting device. A thin, flexible transparent sheath encloses the camera cover. A cell extraction device cover encloses the cell extraction device. The location of the cervix is determined by utilizing the camera. The cell extraction device is pushed outward from the cell extraction device cover so that said cell extraction device head contacts the cervix and removes cells from the cervix. The cell extraction device is pulled back into the cell extraction device cover after the cells have been removed from the cervix. In a preferred embodiment the camera and light emitting device is an endoscope and the camera cover is an endoscope cover.
Owner:MILLARD MATTHEW D +2
Method for detecting pathogenic agents
InactiveUS20050020937A1Accurate detectionMicrobiological testing/measurementSurgerySelf samplingHpv testing
The invention provides methods and kits for collection of cervical / vaginal specimen. The specimen is collected for the purpose of detecting the presence or absence of etiological agents, e.g., bacterial or viral agents, including but not limited to human papilloma virus (HPV). The endocervical cells is not required for HPV testing or Pap smear. The methods and kits allow the self-sampling without the aid of trained medical personnel.
Owner:WEST VIRGINIA UNIVERSITY
Method for preparing pulled type cervical smear
InactiveCN103471892AHigh sample satisfactionNo overlapping stackingPreparing sample for investigationLiquid base cytologyHuman papillomavirus
The invention relates to a method for preparing a pulled type cervical smear. The method comprises the following steps: (1) oscillating a specimen through an oscillator; (2) centrifugating a part of the oscillated specimen until the specimen is layered into supernatant liquid and a cell layer; (3) removing the supernatant liquid, sucking the cell layer, dripping the cell layer on a glass slide, laminating the glass slide with another glass slide, and then pulling the glass slides to the opposite directions; (4) fixing and dyeing so as to prepare the smear. Compared with a conventional Pap smear method, the method provided by the invention has the advantages of high specimen satisfaction, high smear quality and the like. Compared with a liquid base cytology method, the method provided by the invention has the advantages of simplicity in operation, uniform tiling of the prepared smears, non-overlapping of cells, clear background, no precious equipment, low cost and the like, and is convenient to popularize. Compared with a human papillomavirus-deoxyribose nucleic acid (HPV-DNA) detection method, the method provided by the invention has the advantages of no use of precious equipment, low inspection cost, simplicity in operation, convenience in popularization and the like.
Owner:THE FIRST AFFILIATED HOSPITAL OF XIAMEN UNIV
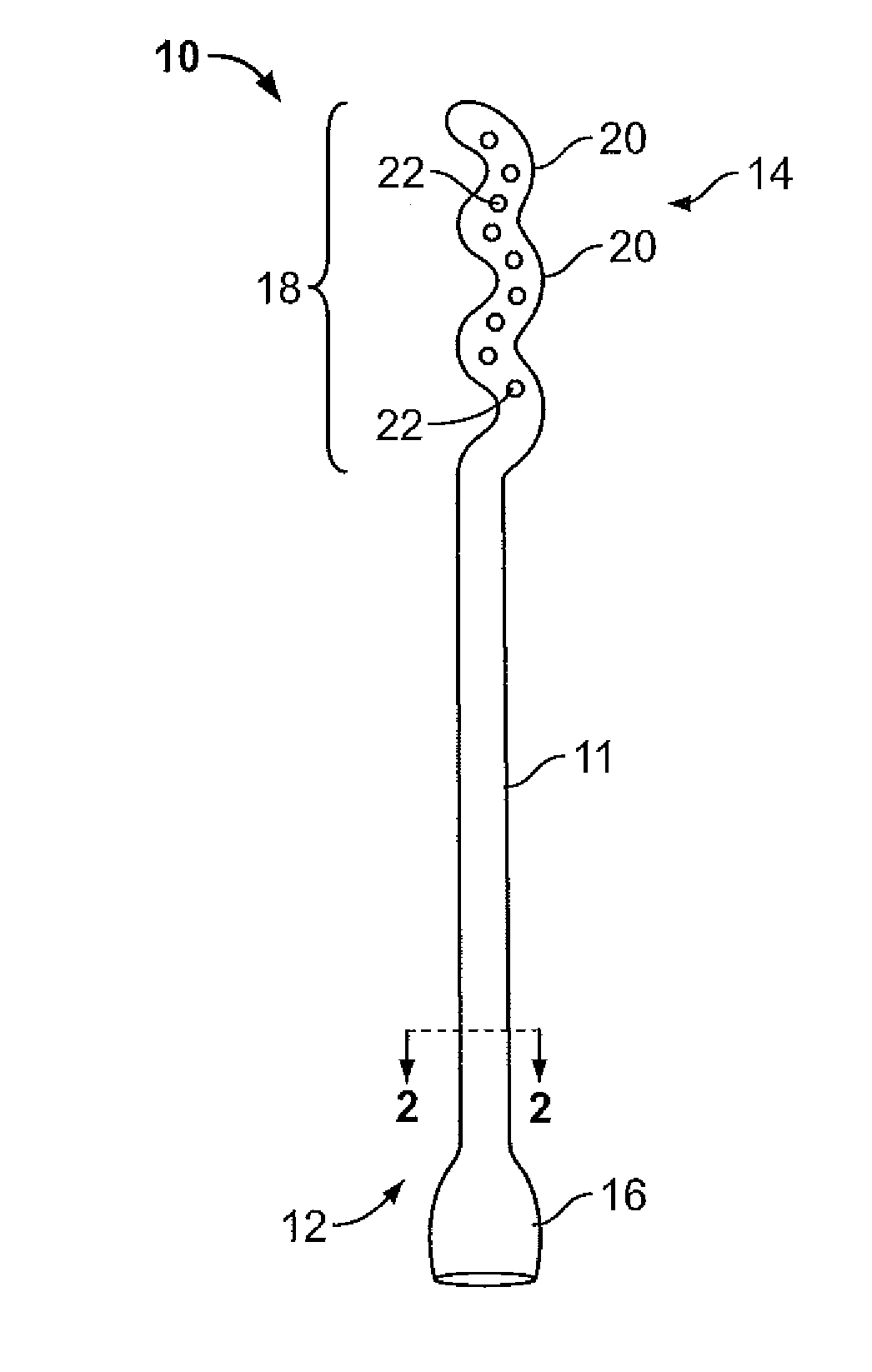
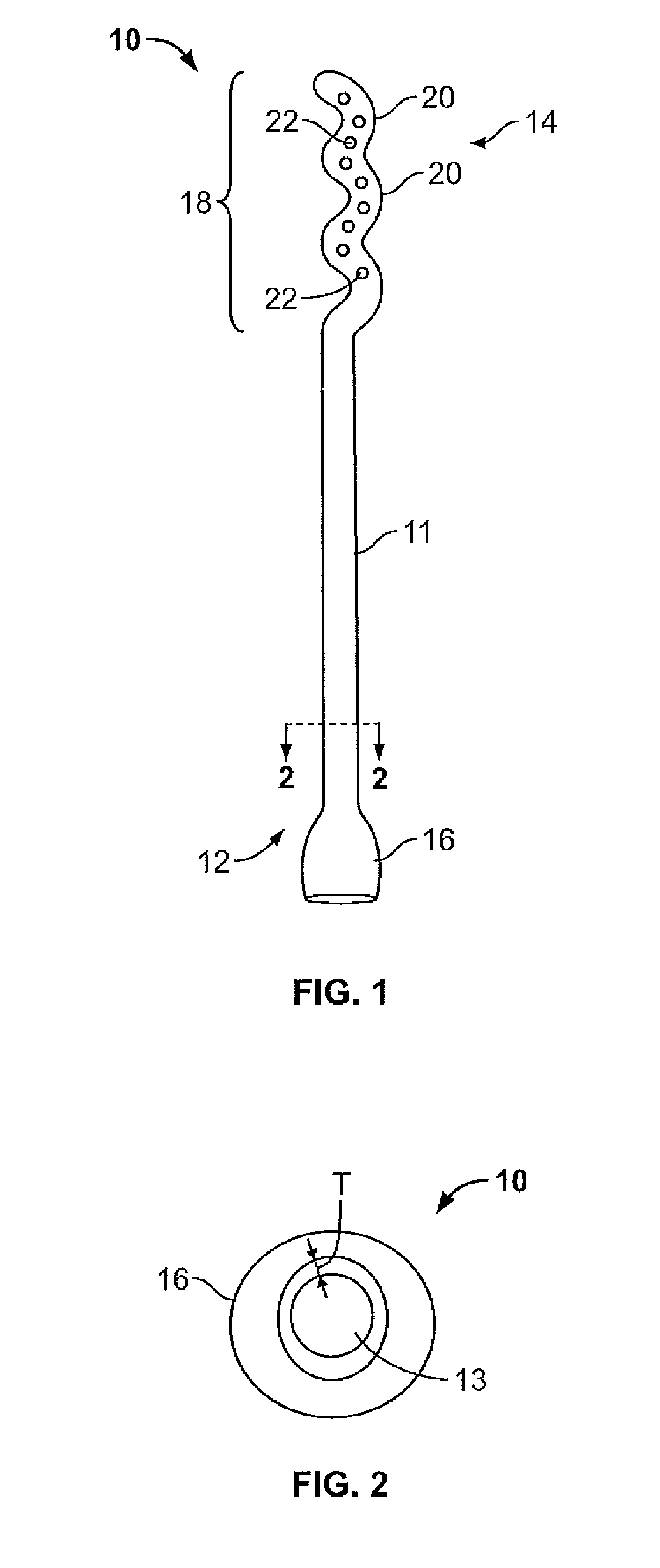
Vagina Probe with Brush
ActiveUS20180078242A1Less discomfortAlleviate potential injurySurgical needlesVaccination/ovulation diagnosticsPap smearsVagina
A sampling probe, having a housing, with a flange at housing's proximal end, and an internal step ring including a protruding follower tab toward housing's distal end. The sampling probe further includes a shaft inside the housing, with a brush at shaft's distal end and a handle at shaft's proximal end. The shaft includes a solid proximal section, and a spirally grooved distal section, engaging the housing's follower tab. A retracting compression spring is positioned on the spirally grooved distal section of the shaft inside the housing. With the sampling probe distally inserted in a vagina canal for a Pap smear, pushing the shaft handle distally, the brush at the end of the shaft protrudes out of the housing rotationally, taking a cell sample, followed by the retracting compression spring retracting the brush back into the housing when the shaft handle is released, completing the Pap smear.
Owner:AGHDAM LIDA
Device and method for conducting a pap smear test
ActiveUS20170119240A1Surgical navigation systemsVaccination/ovulation diagnosticsCell extractionPap smears
A protective guide for protecting a cell extraction device during a Pap smear. A camera protective cover encloses a camera and a light emitting device. A thin, flexible transparent sheath encloses the camera cover. A cell extraction device cover encloses the cell extraction device. The location of the cervix is determined by utilizing the camera. The cell extraction device is pushed outward from the cell extraction device cover so that said cell extraction device head contacts the cervix and removes cells from the cervix. The cell extraction device is pulled back into the cell extraction device cover after the cells have been removed from the cervix. In a preferred embodiment the camera and light emitting device is an endoscope and the camera cover is an endoscope cover.
Owner:MILLARD MATTHEW D +2
Cervical carcinoma screening method
ActiveCN103558150ANo bloodReduce overlapSurgical needlesMaterial analysis by optical meansEarly carcinomaDisease
The invention provides a cervical carcinoma screening method which employs an improved Pap smear for screening. The method comprises the steps: extracting uterine neck secretion, performing conventional staining, film reading and diagnosis; if a patient is diagnosed as ASCUS or a more serious disease by improved Pap smear in cytologic level, performing pathology biopsy under iodine staining inspection. The invention also provides a cervical carcinoma two-method combined screening method. The method comprises: acquiring uterine neck secretion by improved Pap smear, immediately employing an acetic-acid naked-eye inspection method for screening: if a patient is diagnosed as positive by the acetic-acid naked-eye inspection method, performing pathology biopsy at once; and if a patient is diagnosed as negative, waiting for a diagnosis result of the improved Pap smear on site, if the patient is diagnosed as ASCUS or a more serious disease by the improved Pap smear, performing pathology biopsy at once. The methods provided by the invention employ two, but not one, methods for screening on women at the same time, which is not achieved before the methods provided by the invention are disclosed. The methods provided by the invention help to improve detection rate of cervical precancerous lesion and early-stage cancer.
Owner:SHIHEZI UNIVERSITY
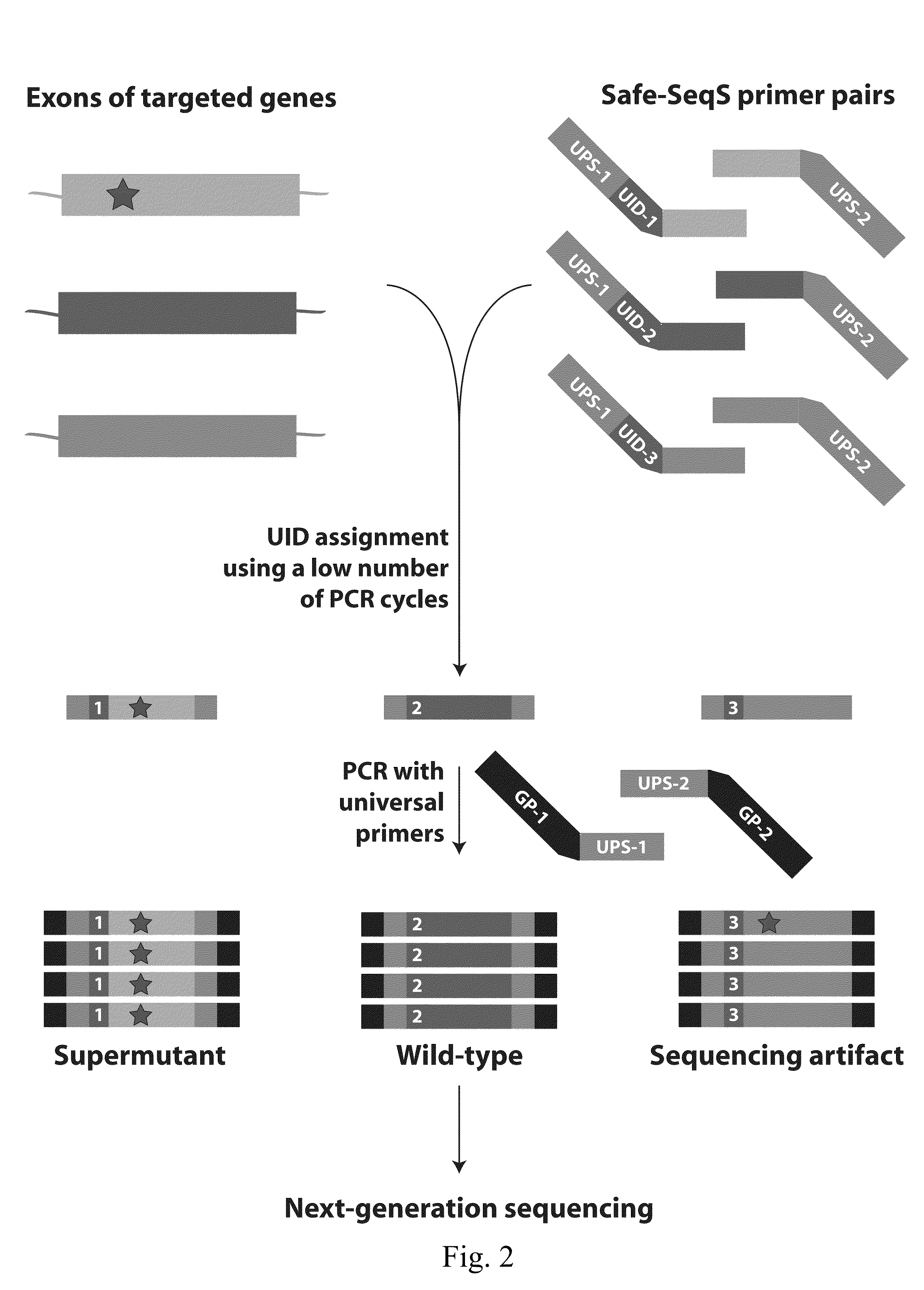
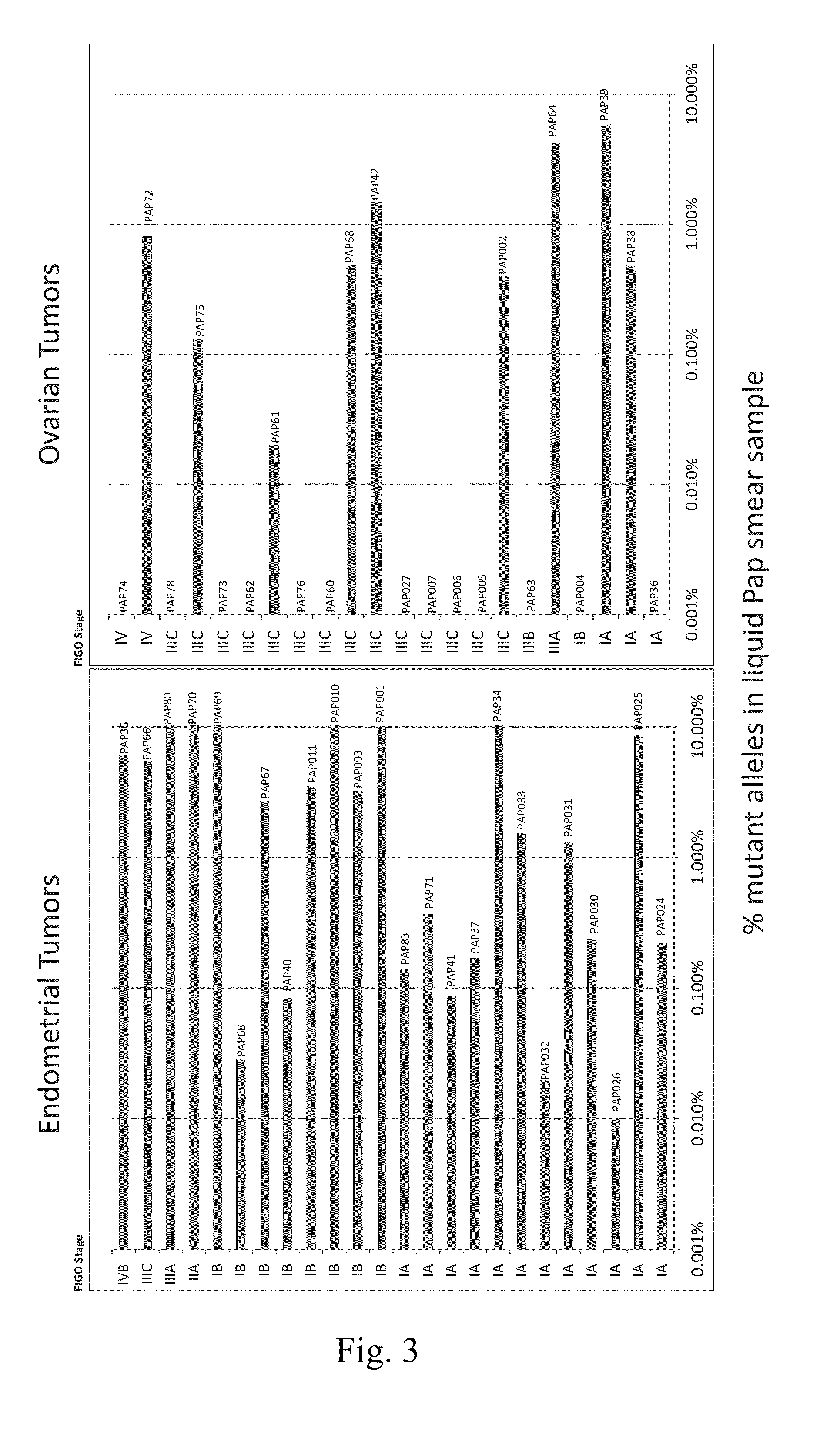
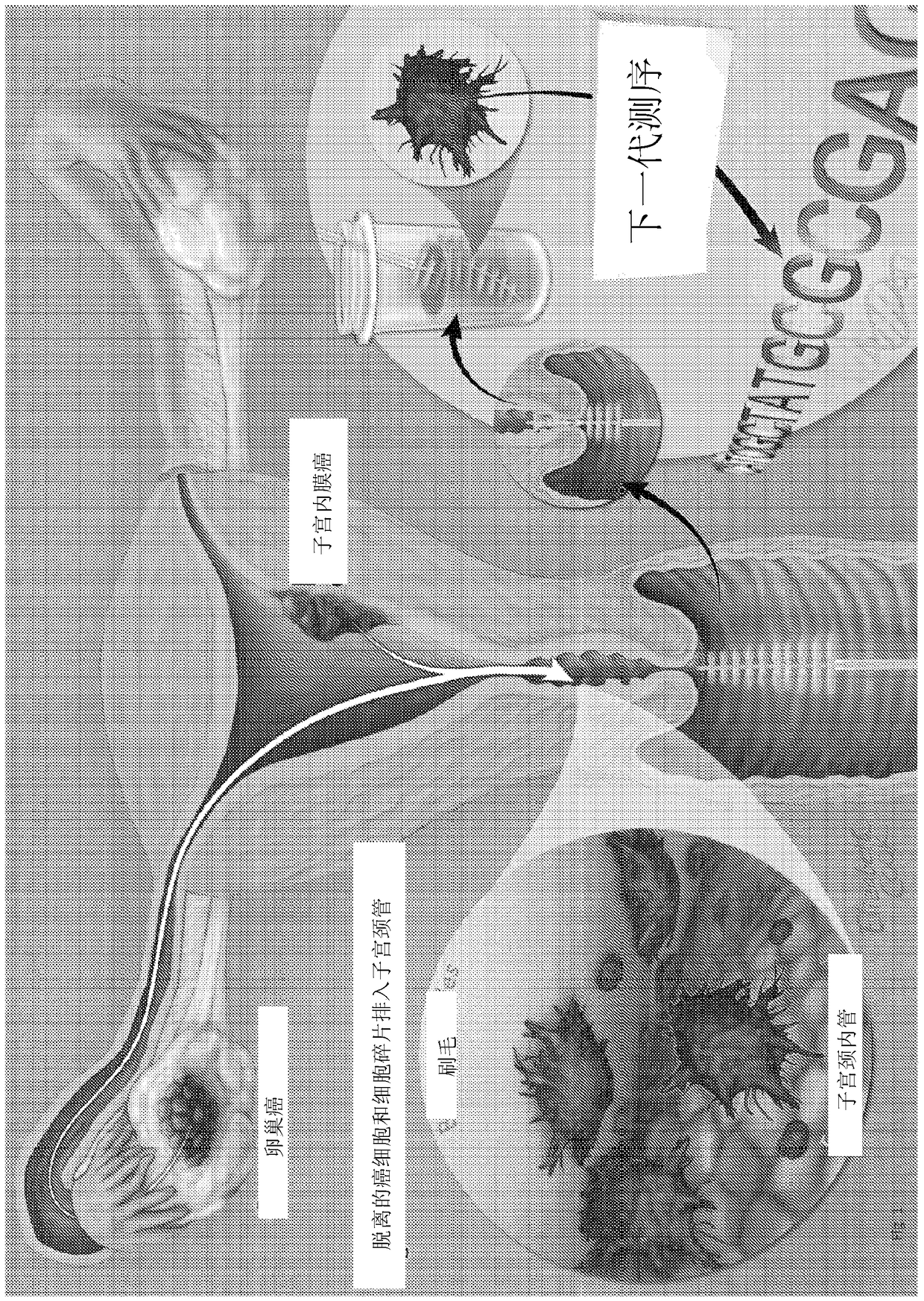
Papanicolaou test for ovarian and endometrial cancers
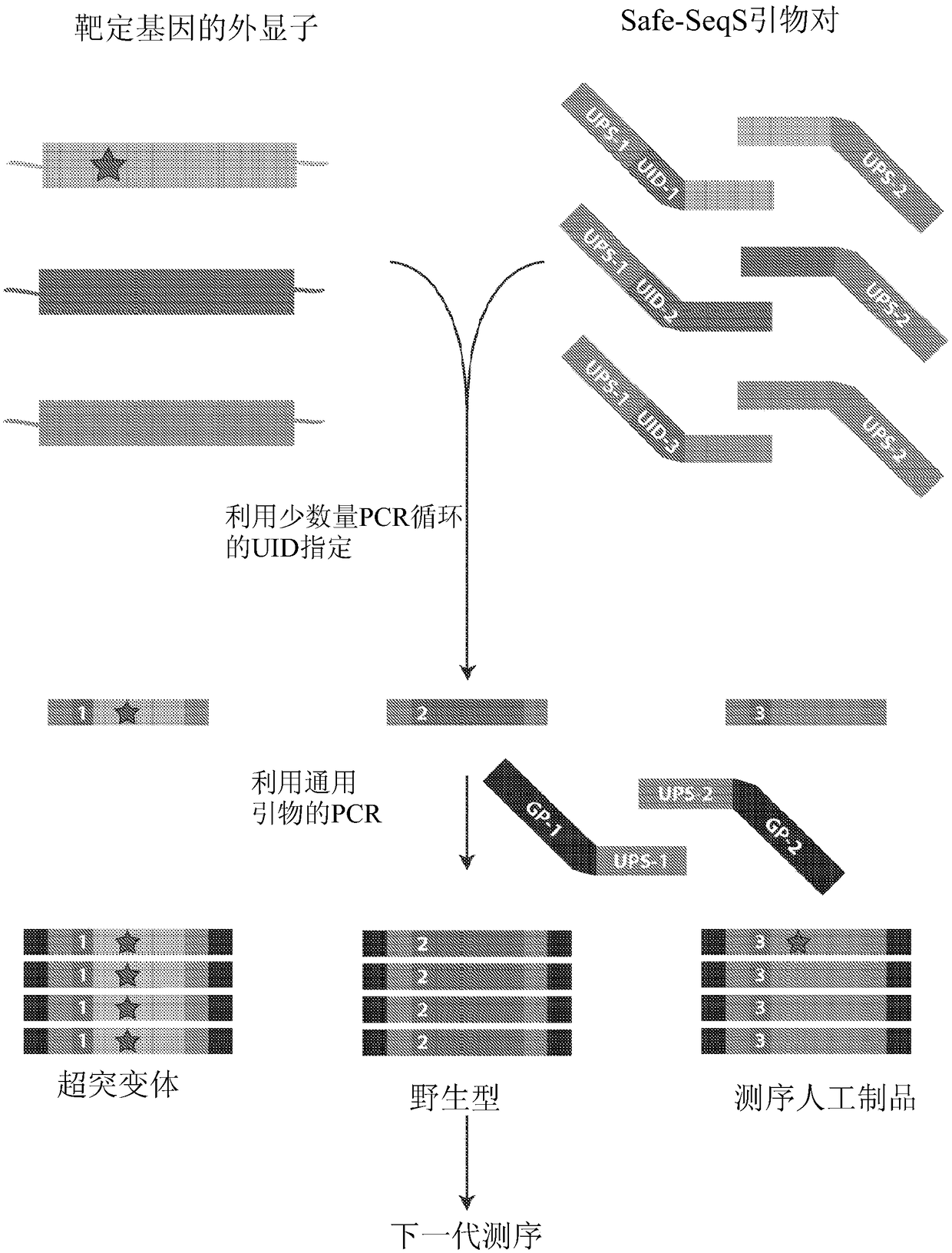
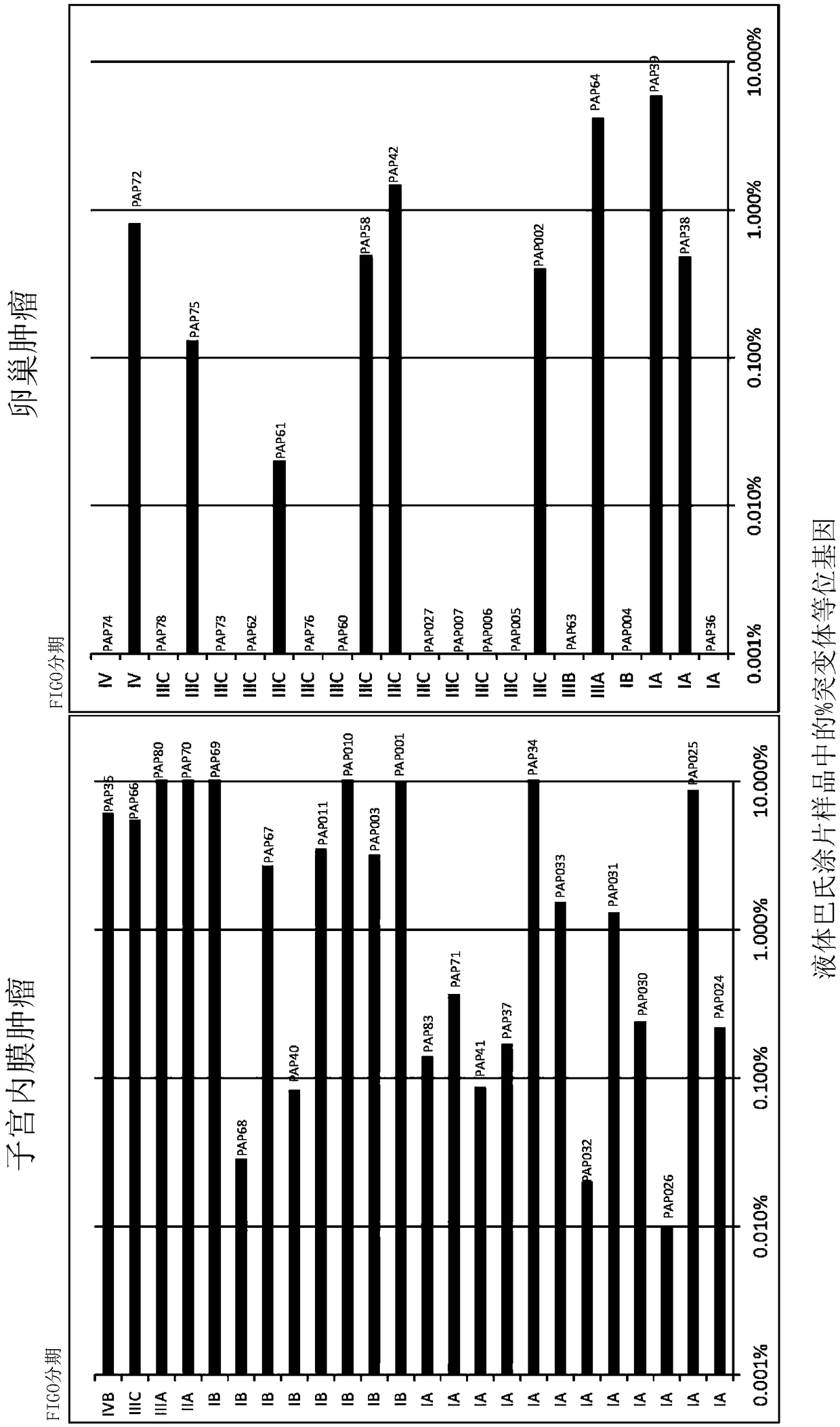
The recently developed liquid-based Papanicolaou (Pap) smear allows not only cytologic evaluation but also collection of DNA for detection of HPV, the causative agent of cervical cancer. We tested these samples to detect somatic mutations present in rare tumor cells that might accumulate in the cervix once shed from endometrial and ovarian cancers. A panel of commonly mutated genes in endometrial and ovarian cancers was assembled and used to identify mutations in all 46 endometrial or cervical cancer tissue samples. We were able also able to identify the same mutations in the DNA from liquid Pap smears in 100% of endometrial cancers (24 of 24) and in 41% of ovarian cancers (9 of 22). We developed a sequence-based method to query mutations in 12 genes in a single liquid Pap smear without prior knowledge of the tumor's genotype.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Endocyte cannula
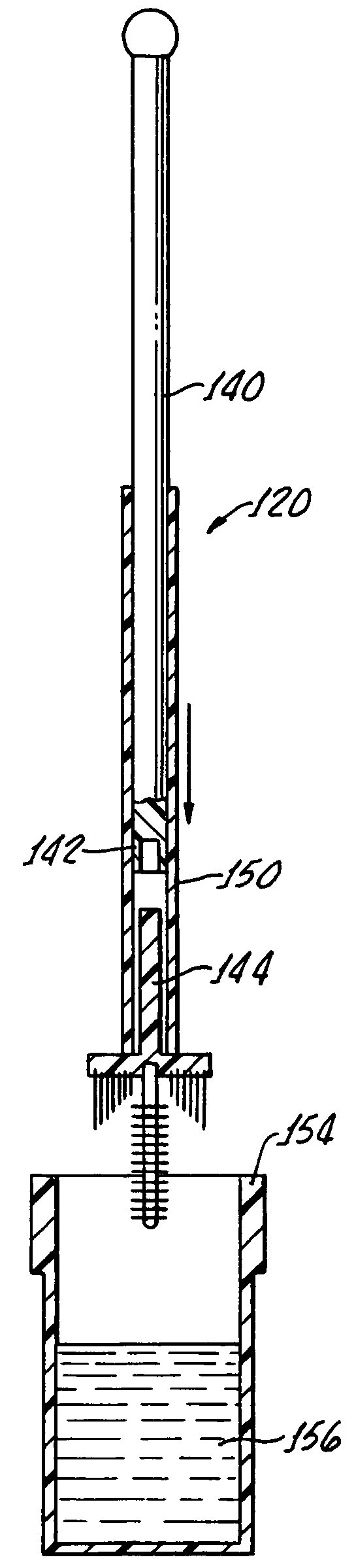
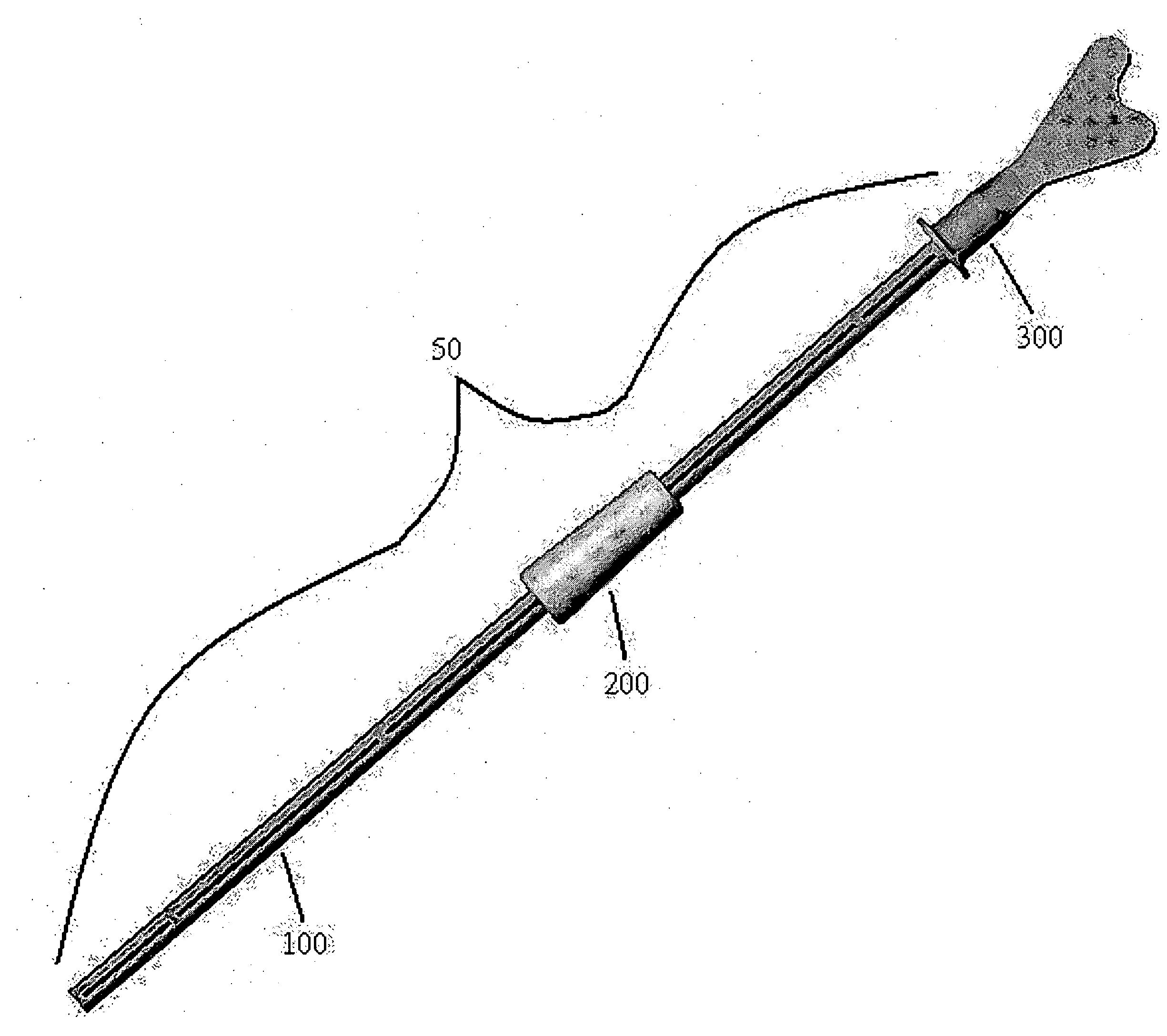
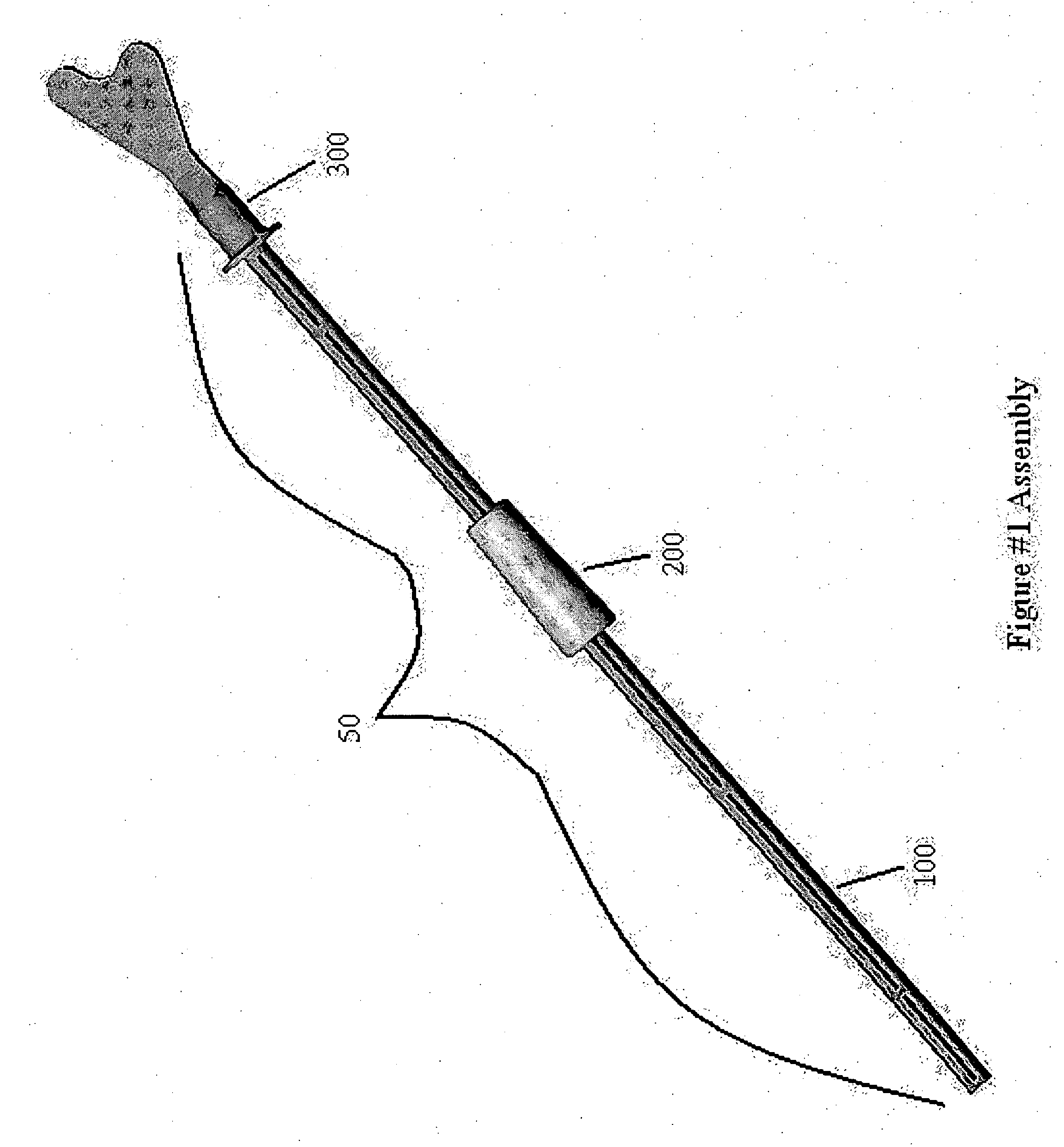
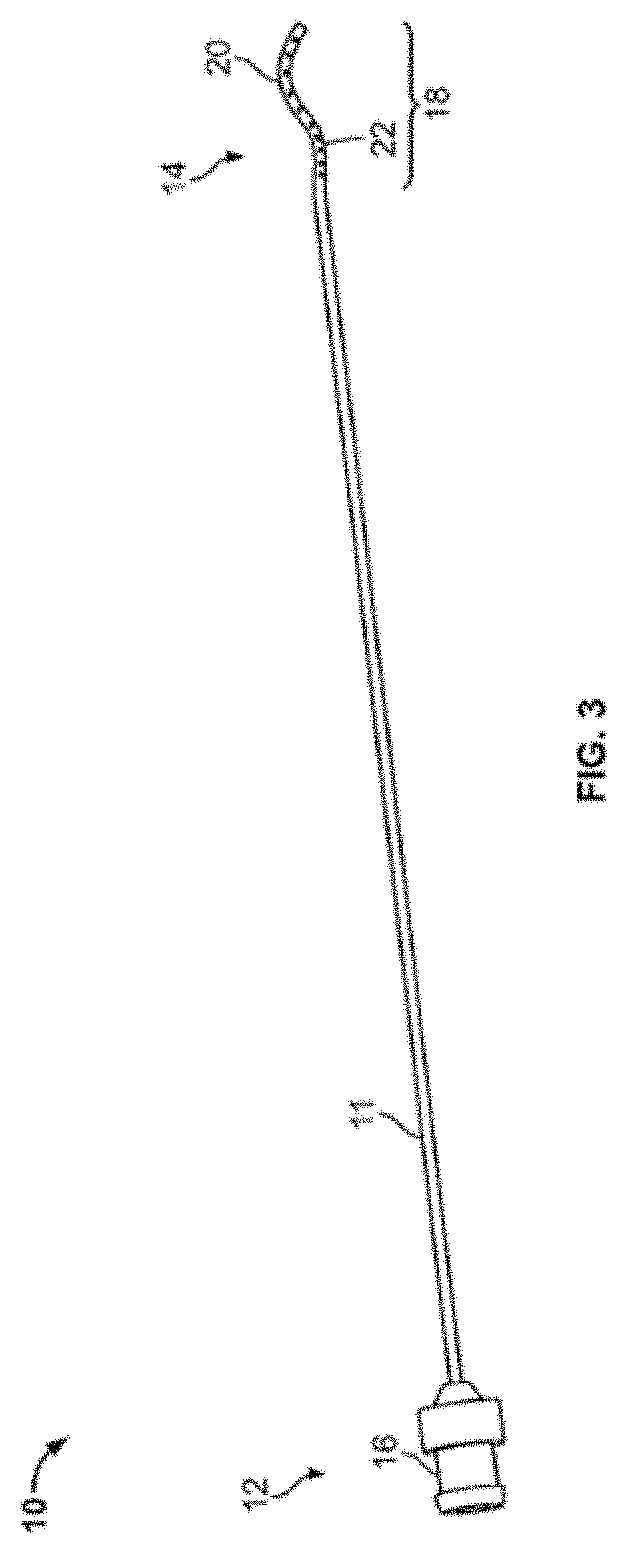
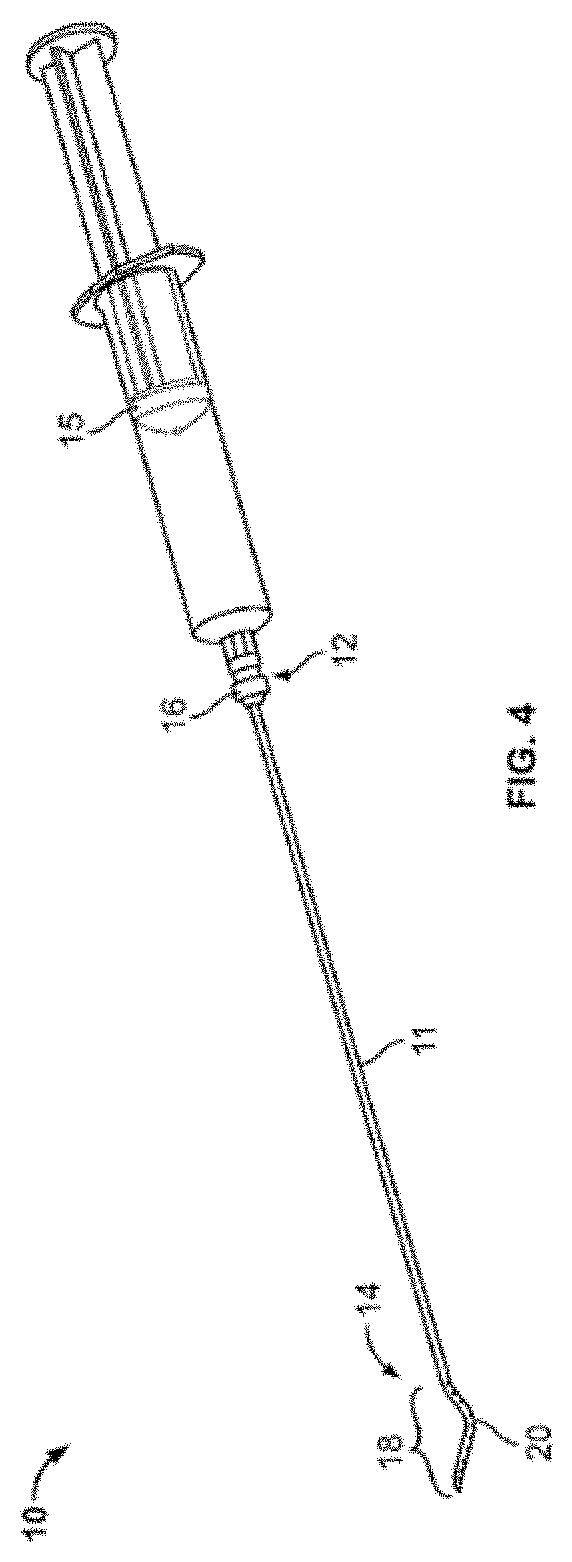
InactiveUS20170007216A1Improved and more productivePromote sportsSurgical needlesVaccination/ovulation diagnosticsDistal portionSurgery
An endocyte cannula is designed to provide improved and more productive Pap smear tests. The endocyte cannula includes a proximal portion and a distal portion. The proximal portion may include a fitting to connect to a user-actuated tool, such as a syringe. The distal portion may include a corkscrew feature to facilitate movement of the cannula through dense tissue. The distal portion may further include a plurality of apertures to aspirate mucus and reduce the risk of trauma or perforation.
Owner:ALL CAPE GYNECOLOGY
Hypermethylation Biomarkers for Detection of Cervical Cancer
ActiveUS20130109584A1Nucleotide librariesMicrobiological testing/measurementParanasal Sinus CarcinomaEpigenetic Profile
Pap smears and HPV infection tests do not distinguish between lesions that will progress to an invasive carcinoma and those that will not. We aimed to identify epigenetic biomarkers for diagnosis and progression monitoring of premalignant lesions in cervical cancer. Hypermethylated genes were identified as potential biomarkers after validation by MSP, including GGTLA4 and ZNF516. The methylation frequency for these two genes was higher in tumor: GGTLA4 (100%) and ZNF516 (96%); than in normal samples: GGTLA4 (12%) and ZNF516 (16%). The methylation status of GGTLA4 showed a progression in methylation frequency from normal samples to invasive carcinoma. The immunohistochemical expression was lower in tumor for both: GGTLA4 (50.8%) and ZNF516 (66.2%); than in normal samples: GGTLA4 (71.2%) and ZNF516 (88.1%) (p<0.05). In conclusion, we identified methylation biomarkers for the molecular screening and characterization of cervical cancer.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Intelligent high-throughput tumor screening and analyzing platform
InactiveCN110313935AStrive for early detectionEffective treatmentUltrasonic/sonic/infrasonic diagnosticsGastroscopesInfraredTreatment effect
The invention discloses an intelligent high-throughput tumor screening and analyzing platform. The platform comprises a blood checking device, a B-type ultrasonic detecting device, a PET-CT diagnosticinstrument, a gynecological physical examination pap smear detector, a gastroscope device, an enteroscope device, a tumor marker screening and detecting device, a bone scanning device and an infraredscanner, wherein the blood examination device, the B-type ultrasonic detection device, the PET-CT diagnostic instrument, the gynecological physical examination pap smear detector, the gastroscope device, the enteroscope device, the tumor marker screening and detecting device, the bone scanning device and the infrared scanner are all connected with a computer detection analysis unit through signallines. By adopting the technical scheme, tumor examination results can be rapidly, comprehensively and accurately obtained at the early stage of tumors, the screening efficiency is improved to find the tumors as early as possible, it is ensured that the tumors are effectively treated in the early stage, the diagnosis and treatment effects are improved, and pain of patients and family members is relieved.
Owner:安徽健朗医疗器械有限公司
DNA hypermethylation of promoters of target genes and clinical diagnosis and treatment of HPV related disease
InactiveUS20150051100A1Nucleotide librariesMicrobiological testing/measurementDiseaseCervical tissue
The present invention provides arrays for gene loci that allow diagnosis of cervical cancer in patients who may be asymptomatic or have inconclusive Pap smears or cytology, and allowing earlier diagnosis and treatment of the subject. The present invention also provides methods of determination of a global promoter DNA methylation in a cervical tissue sample from a subject, using a variety of methods which can detect DNA methylation. Further, the invention provides methods of diagnosis of cervical cancer in a subject, by comparing the global promoter DNA methylation in a cervical tissue sample obtained from a subject to the global promoter DNA methylation of standard controls. In addition, the present invention also provides a method of diagnosis of cervical cancer in a subject suspected of having cervical cancer after obtaining a biological sample of cervical tissue comprising DNA from the subject and detecting the amount of promoter methylation on at least one or more DNA target sites selected from the group consisting of ZN-F516, INTS1, and FKBP6; and comparing the amount of promoter methylation on at least one or more DNA target sites in the sample of the subject. These methods allow diagnosis of cervical cancer in patients who may be asymptomatic or have inconclusive Pap smears or cytology, and allowing earlier diagnosis and treatment of the subject
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
A kind of preparation method of improved Pap smear
ActiveCN103558150BNo bloodReduce overlapSurgical needlesMaterial analysis by optical meansEarly carcinomaStaining
The invention provides a screening method for cervical cancer, which uses the improved Pap smear method for screening; the steps are: extracting cervical secretions, then carrying out routine staining, film reading, and diagnosis; and improving the Pap smear method for cytological diagnosis For ASCUS or above lesions, pathological biopsy should be performed immediately under iodine staining. The present invention also provides a screening method for cervical cancer. After the cervical secretions are obtained by the improved Pap smear method, the acetic acid macroscopic examination is immediately used for screening. If the acetic acid visual examination is positive, pathological biopsy is taken If the diagnosis is negative, wait for the diagnosis result of the modified Pap smear method at the scene, and if the modified Pap smear method is diagnosed as ASCUS or above lesions, a pathological biopsy is taken immediately. The present invention screens women with two methods at the same time, not one. Before this invention, this was not possible. The method of the invention can improve the detection rate of cervical precancerous lesion and early cancer.
Owner:SHIHEZI UNIVERSITY
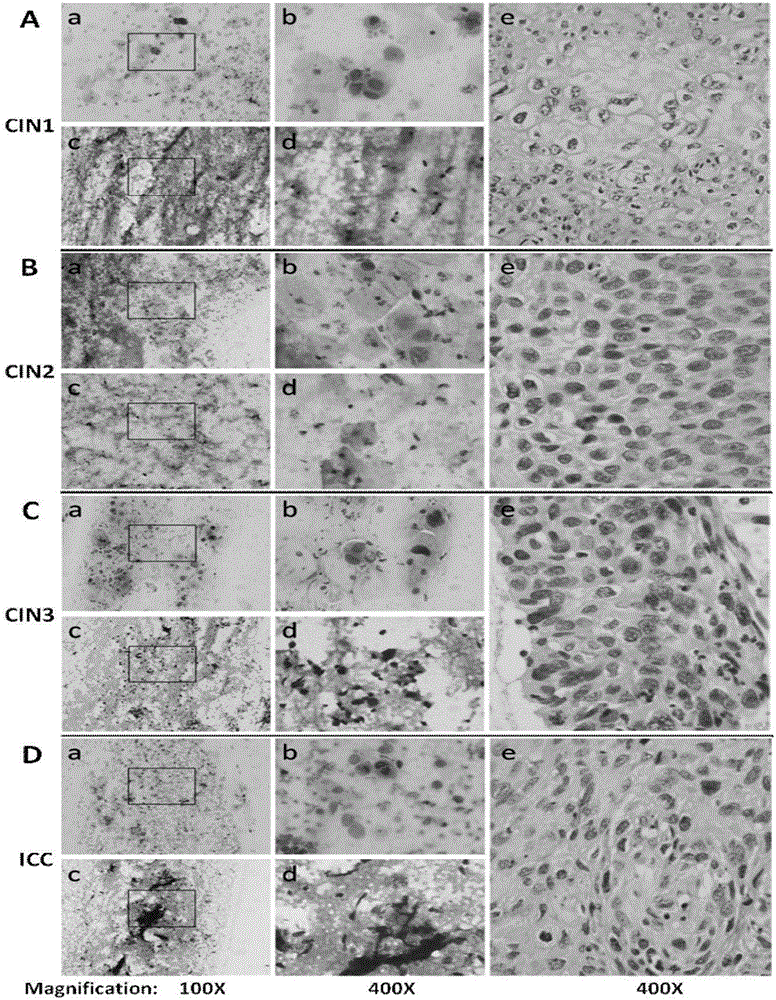
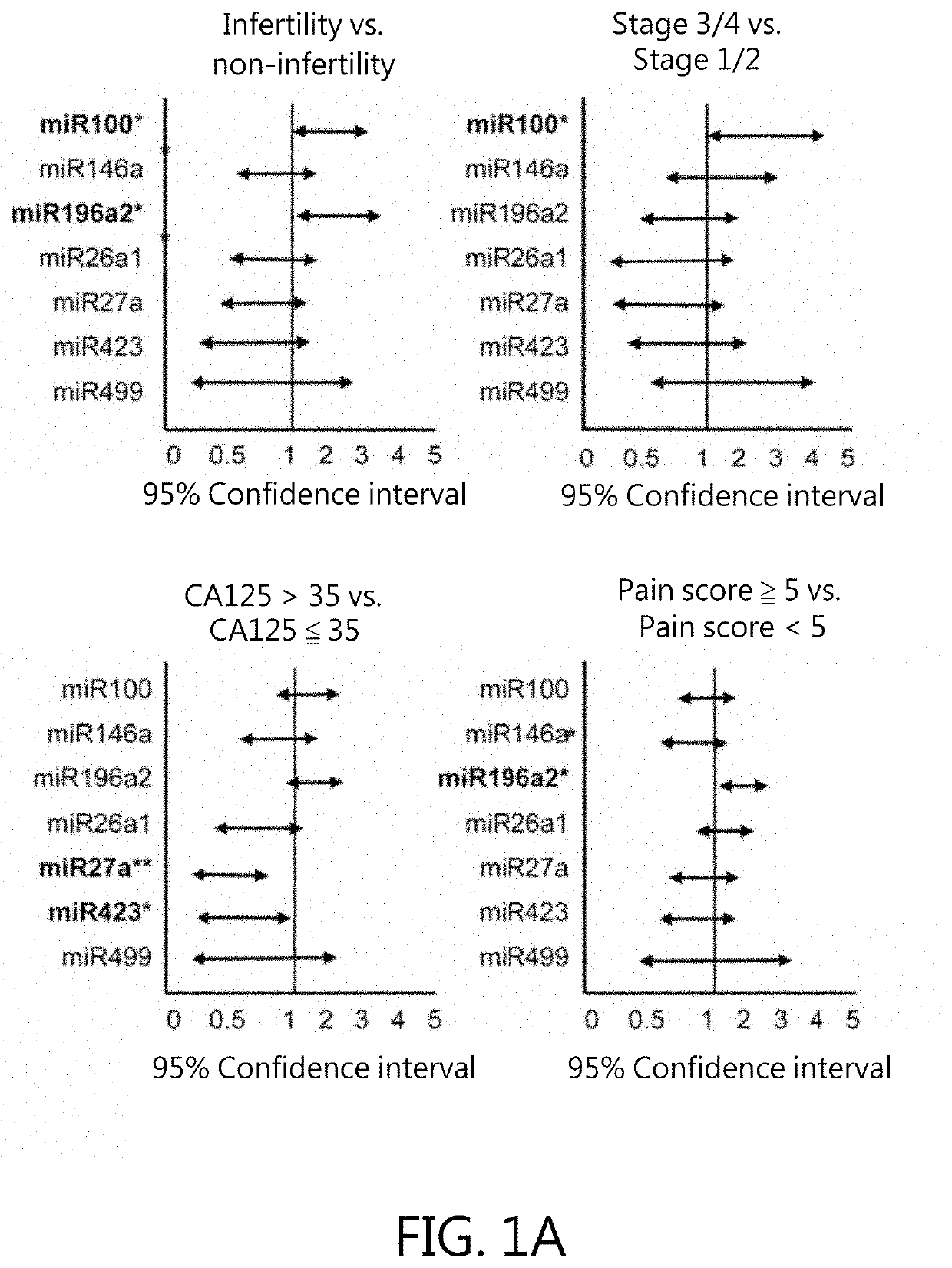
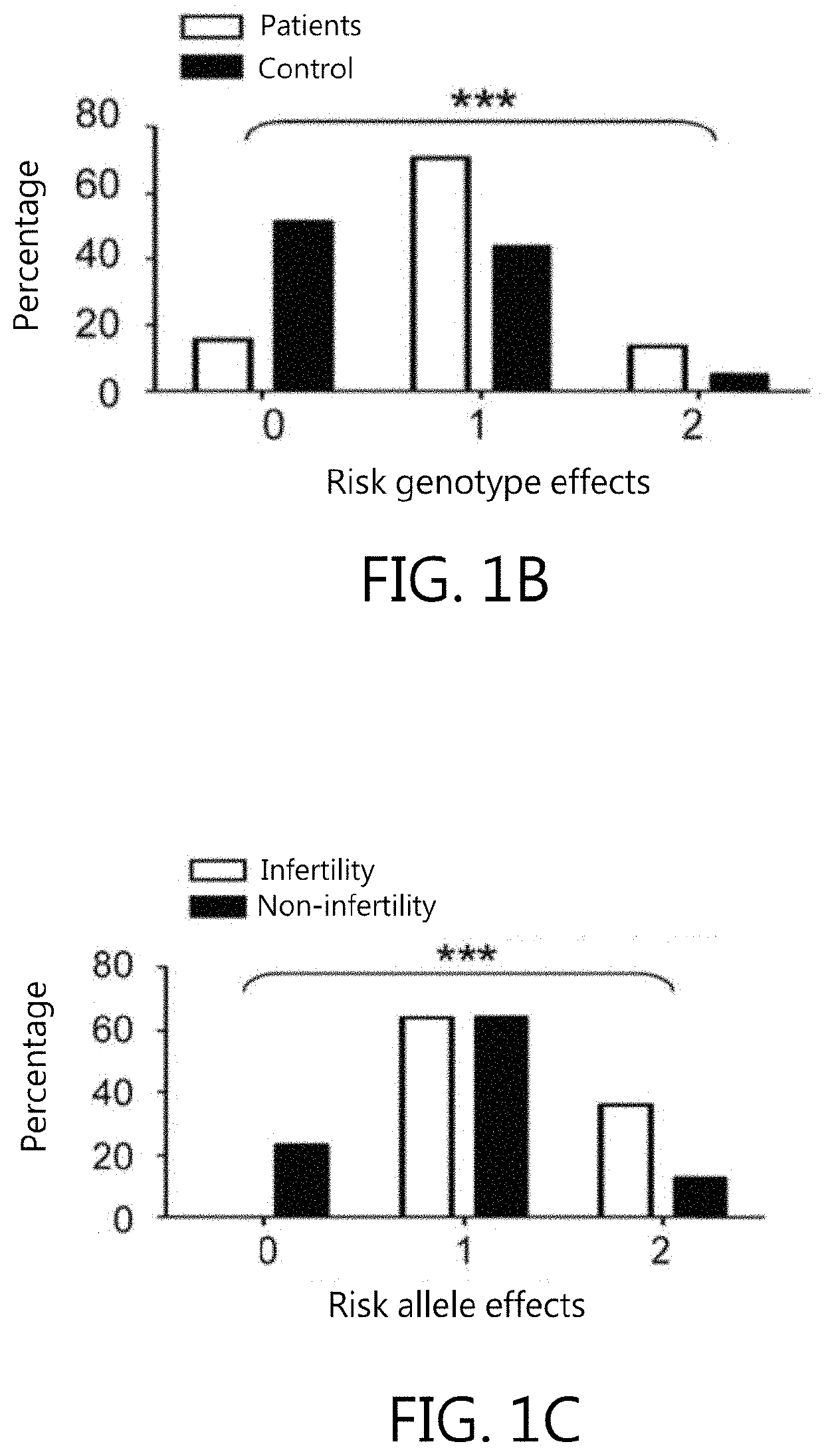
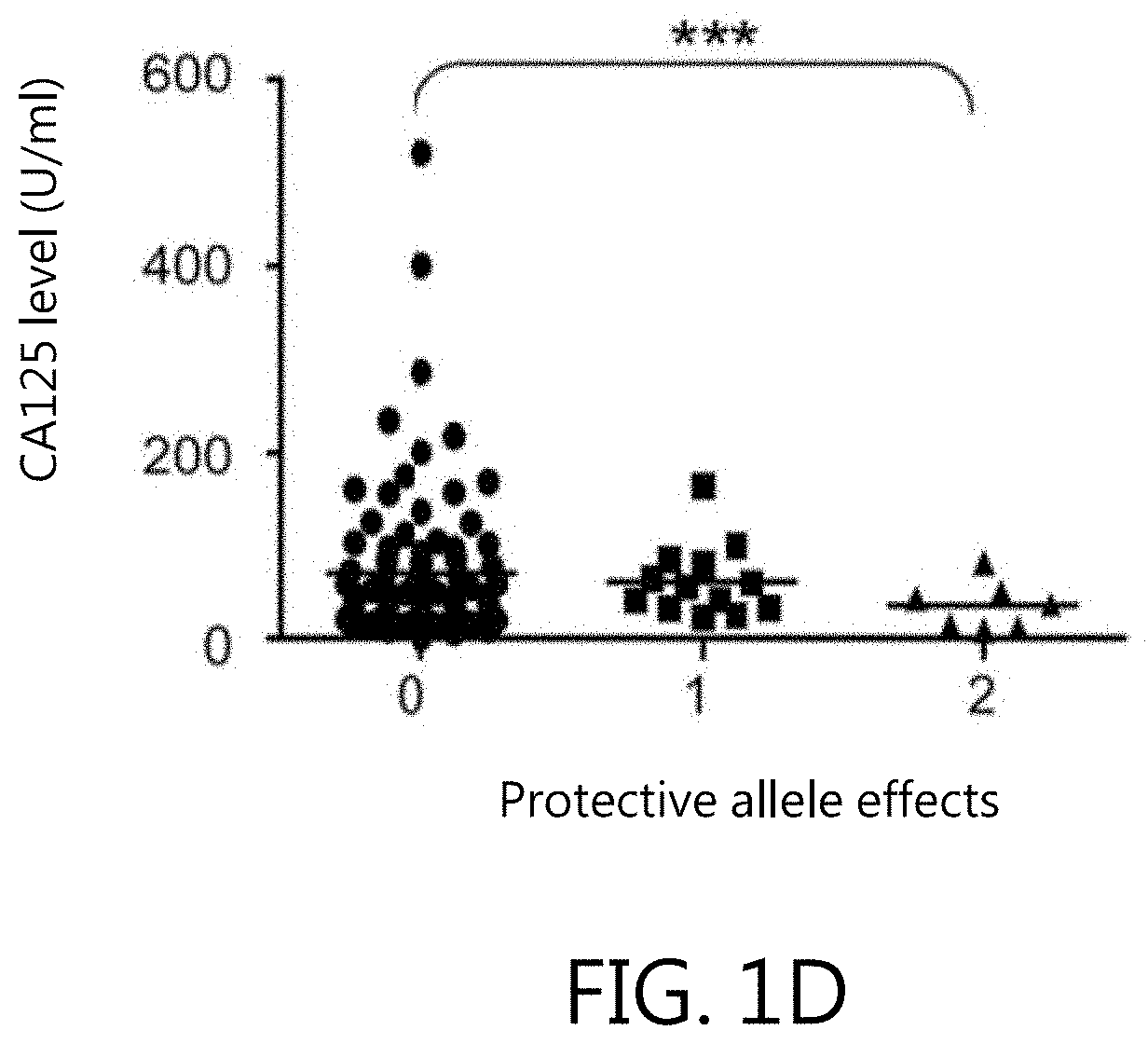
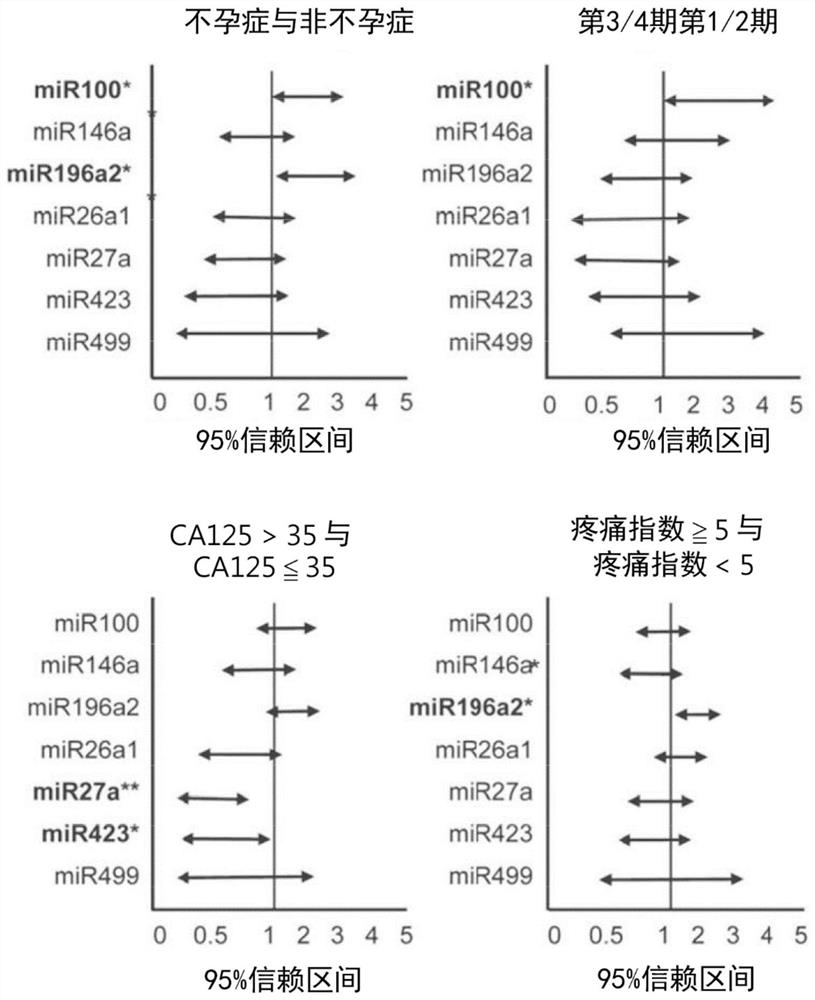
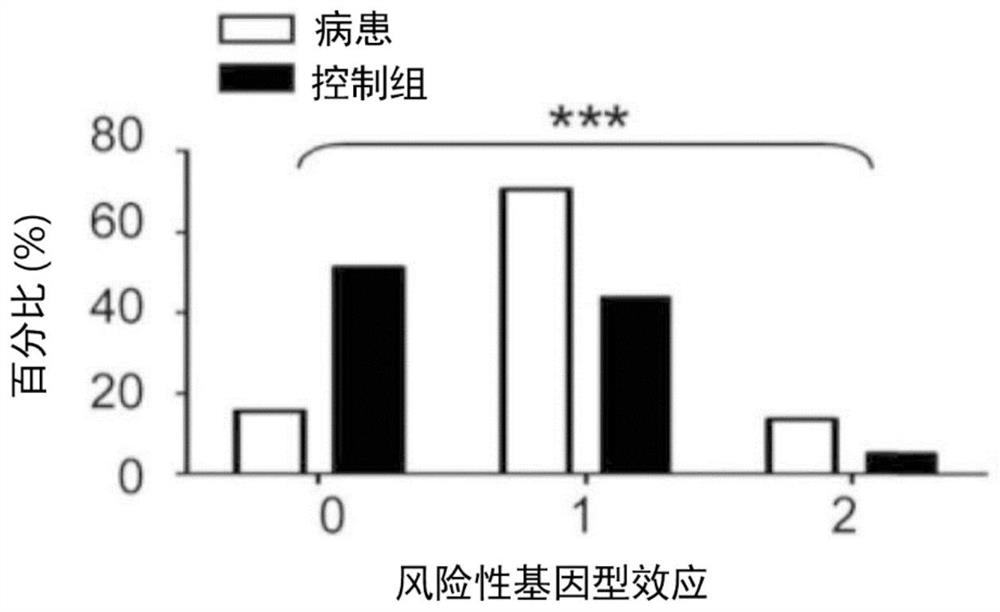
Kit for in vitro testing panel of genes in pap smear samples for endometriosis and method of non-invasively and qualitatively determining severity of endometriosis using the kit
The present invention relates to a kit for in vitro testing a panel of genes in Pap smear samples for endometriosis and a method of non-invasively and qualitatively determining severity of endometriosis using the kit. A biomarker and a probe used in the kit and the method include a specific SNP site and a product expressed by a gene related to the SNP site, for detecting the genotype of SNP site and the product expressed by the gene corresponding to the SNP site, so as to accurately determine the severity of endometriosis. The biomarker and the probe can be applied to a kit for in vitro testing a panel of genes in Pap smear samples for endometriosis.
Owner:CHINA MEDICAL UNIVERSITY(TW)
Endocyte cannula
PendingUS20210100539A1Improved and more productive Pap smear testPromote sportsSurgical needlesVaccination/ovulation diagnosticsDistal portionEndocervical canal
An endocyte cannula is designed to provide improved and more productive Pap smear tests. The endocyte cannula includes a proximal portion and a distal portion. The proximal portion may include a fitting to connect to a user-actuated tool, such as a syringe. The distal portion may include a corkscrew feature or a lateral wing feature to facilitate movement of the cannula through dense tissue as well as provide scraping or brushing capabilities. The shaped scraping or brushing features may be provided on an attachment that is attachable to a shaft of the endocyte cannula. The attachments may allow customization of the endocyte cannula based on the physiology and anatomy of a patient. The distal portion may further include a plurality of apertures to aspirate mucus and reduce the risk of trauma or perforation. The apertures may extend outside of the attachment or shaped scraping or brushing features, and the distal portion may be narrower than the shaped scraping or brushing features to facilitate access into the endocervical canal and aspiration and collection of a cell sample.
Owner:ALL CAPE GYNECOLOGY
Papanicolaou test for ovarian and endometrial cancers
The invention relates to papanicolaou test for ovarian and endometrial cancers. The recently developed liquid-based Papanicolaou (Pap) smear allows not only cytologic evaluation but also collection ofDNA for detection of HPV, the causative agent of cervical cancer. We tested these samples to detect somatic mutations present in rare tumor cells that might accumulate in the cervix once shed from endometrial and ovarian cancers. A panel of commonly mutated genes in endometrial and ovarian cancers was assembled and used to identify mutations in all 46 endometrial or cervical cancer tissue samples. We were able also able to identify the same mutations in the DNA from liquid Pap smears in 100% of endometrial cancers (24 of 24) and in 41% of ovarian cancers (9 of 22). We developed a sequence-based method to query mutations in 12 genes in a single liquid Pap smear without prior knowledge of the tumor's genotype.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Gene detection kit of endometriosis Pap smear sample and method for judging deterioration degree of endometriosis
PendingCN113030482AMicrobiological testing/measurementDisease diagnosisSmear sampleAntiendomysial antibodies
The present invention relates to a gene detection kit of an endometriosis Pap smear sample and a method for judging the deterioration degree of endometriosis. According to the gene detection kit and the in-vitro detection method thereof, at least nine antibodies are used, the specific genotype of the specific SNP site and the expression quantity of at least one of mutually reacted gene products can be qualitatively detected in vitro in a non-invasive manner, and the deterioration degree of endometriosis can be accurately judged; further, the gene detection kit is applied to in-vitro detection of an endometriosis pasteur smear sample.
Owner:SUN YAT SEN UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com