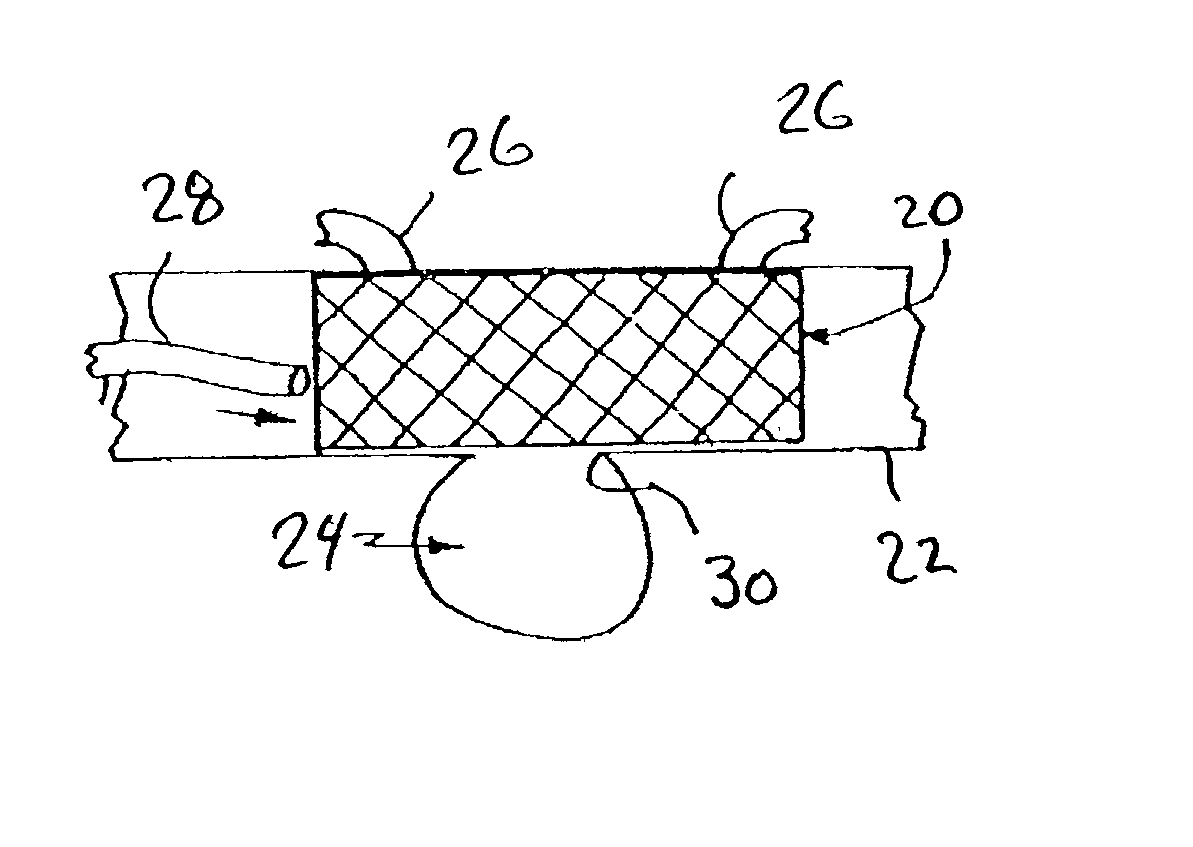
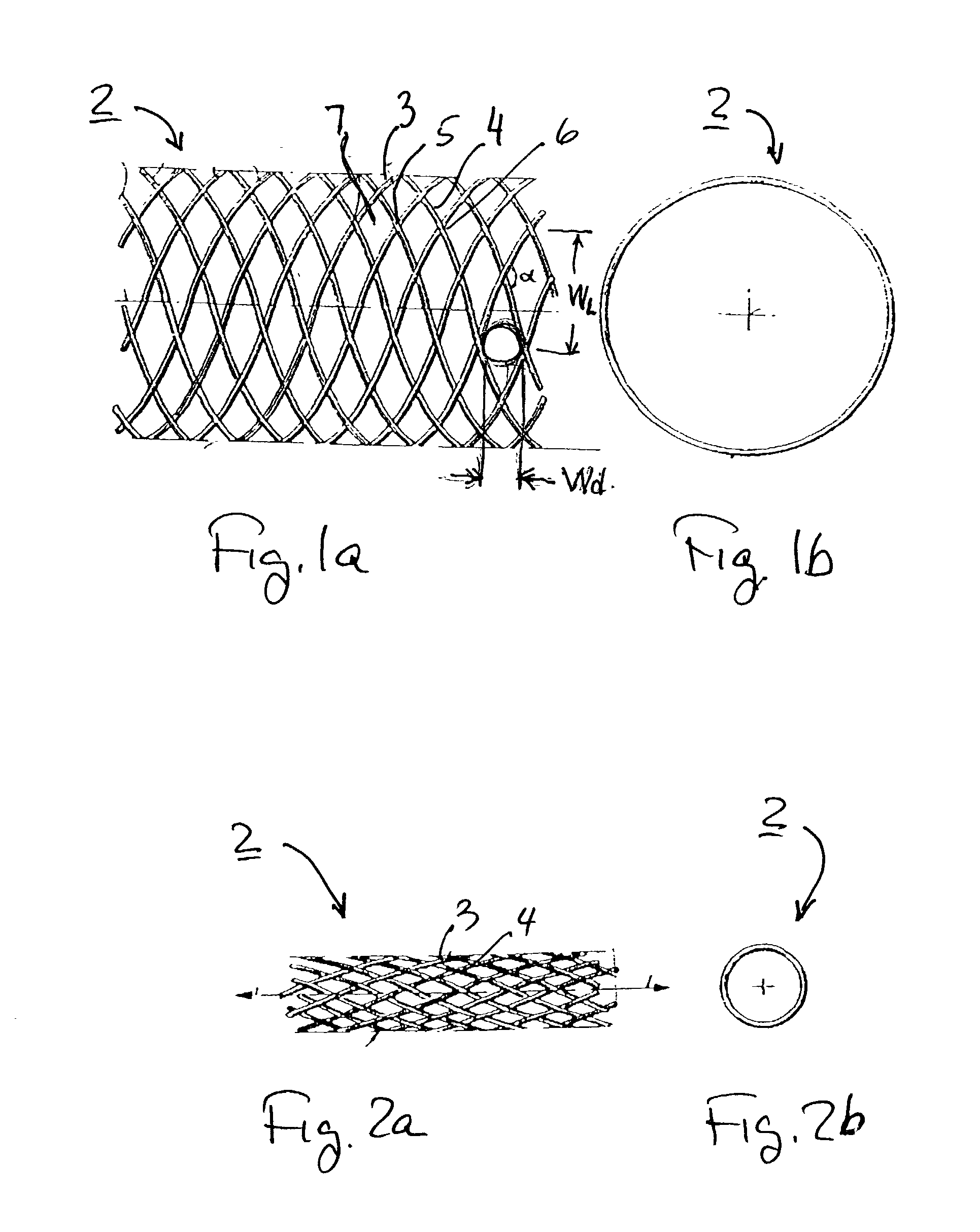
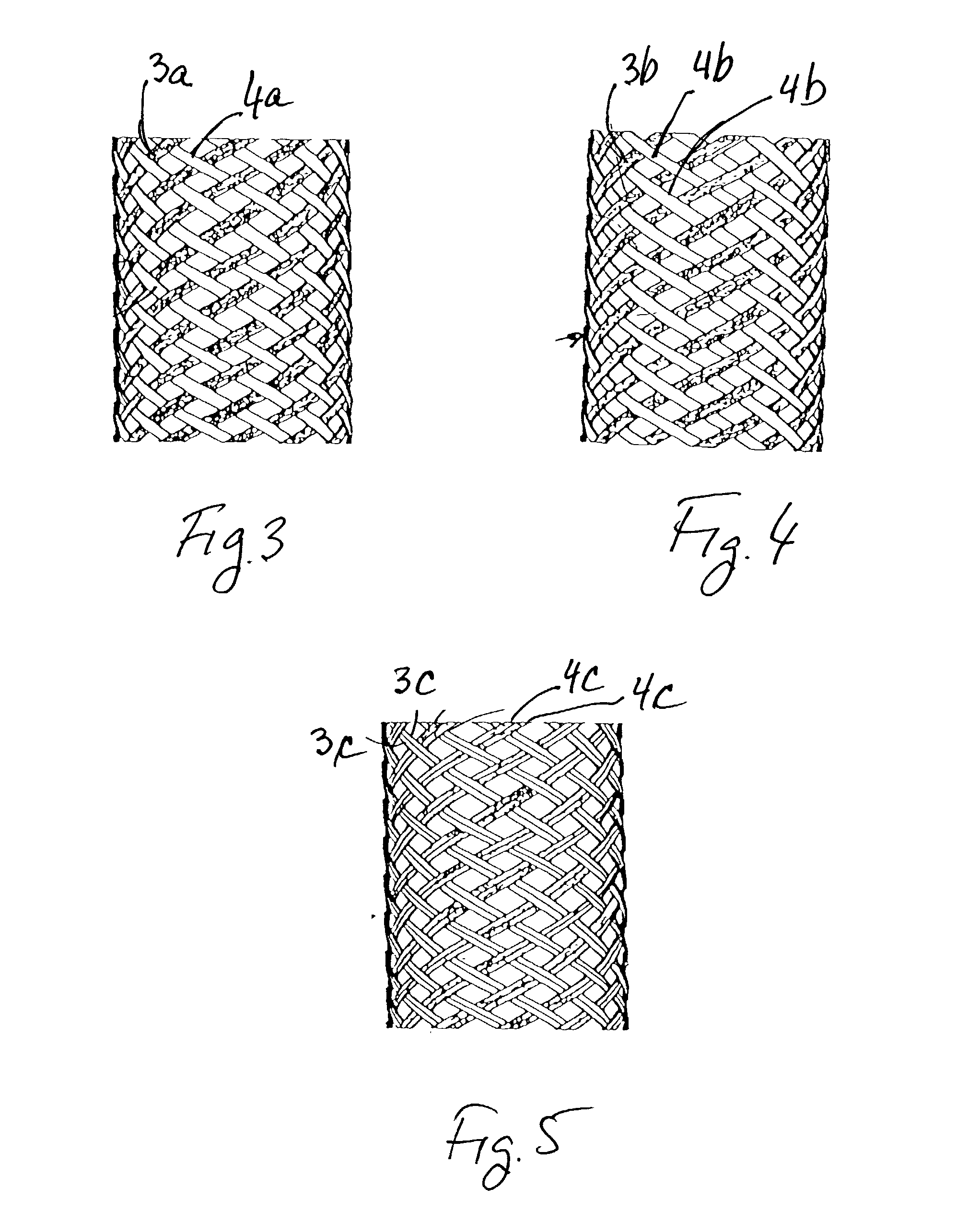
Implantable intraluminal device and method of using same in treating aneurysms
a technology of intraluminal device and aneurysm, which is applied in the field of intraluminal device, can solve the problems of inability to achieve the effect of unable to meet the needs of patients, so as to achieve the effect of maintaining patency in the perforating and/or branch vessels and reducing the blood flow to the aneurysm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0068] Given:
[0069] 1. Artery diameter=3 mm
[0070] 2. Braiding angle .alpha.=90.degree.
[0071] A porosity index of 70% can achieved by changing the filament diameter and the number of filaments as follows:
1 Windows Artery Braiding Number Filaments Inscribed Porosity Diameter Angle of Fil- Width Diameter Index # [mm] [deg] aments [.mu.m] [.mu.m] [%] 1(a) 3 90 108 20 100 70 1(b) 3 90 78 27 140 70 1(c) 3 90 62 35 180 70 Note: For round cross-section filaments, the bending stiffness of #1(a) in its contracted condition is one-fifth that of #1(c) due to the smaller diameter filaments in #1(a).
example 2
[0072] Given:
[0073] 1. Artery diameter=3 mm
[0074] 2. Braiding angle .alpha.=90.degree.
[0075] 3. Filaments diameter=27 .mu.m
[0076] The porosity index can changes by changing the number of filaments as follows:
2 Windows Artery Braiding Number Filament Inscribed Porosity Diameter Angle of Fil- Diameter Diameter Index # [mm] [deg] aments [.mu.m] [.mu.m] [%] 2(a) 3 90 64 27 180 75 2(b) 3 90 108 27 140 70 2(c) 3 90 110 27 80 60 Note: For round cross-section filaments, the bending stiffness of #2(a) in its contracted condition is 60% of #2(c) due to the smaller number of filaments in #2(a).
example 3
[0077] Given:
[0078] 1. Artery diameter=3 mm
[0079] 2. Number of filaments=78
[0080] 3. Filaments diameter=27 .mu.m
[0081] The porosity index can reduced from its maximum value at .alpha.=90.degree. in # 3(a) by changing, i.e., increasing in # 3(b) or decreasing in # 3(c) the braiding angle .alpha. as follows:
3 Windows Artery Braiding Number Filament Inscribed Porosity Diameter Angle of Fil- diameter Diameter Index # [mm] [deg] aments [.mu.m] [.mu.m] [%] 3(a) 3 90 78 27 140 70 3(b) 3 106 78 27 110 65 3(c) 3 56 78 27 110 65
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com