Patents
Literature
331 results about "Self expandable" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
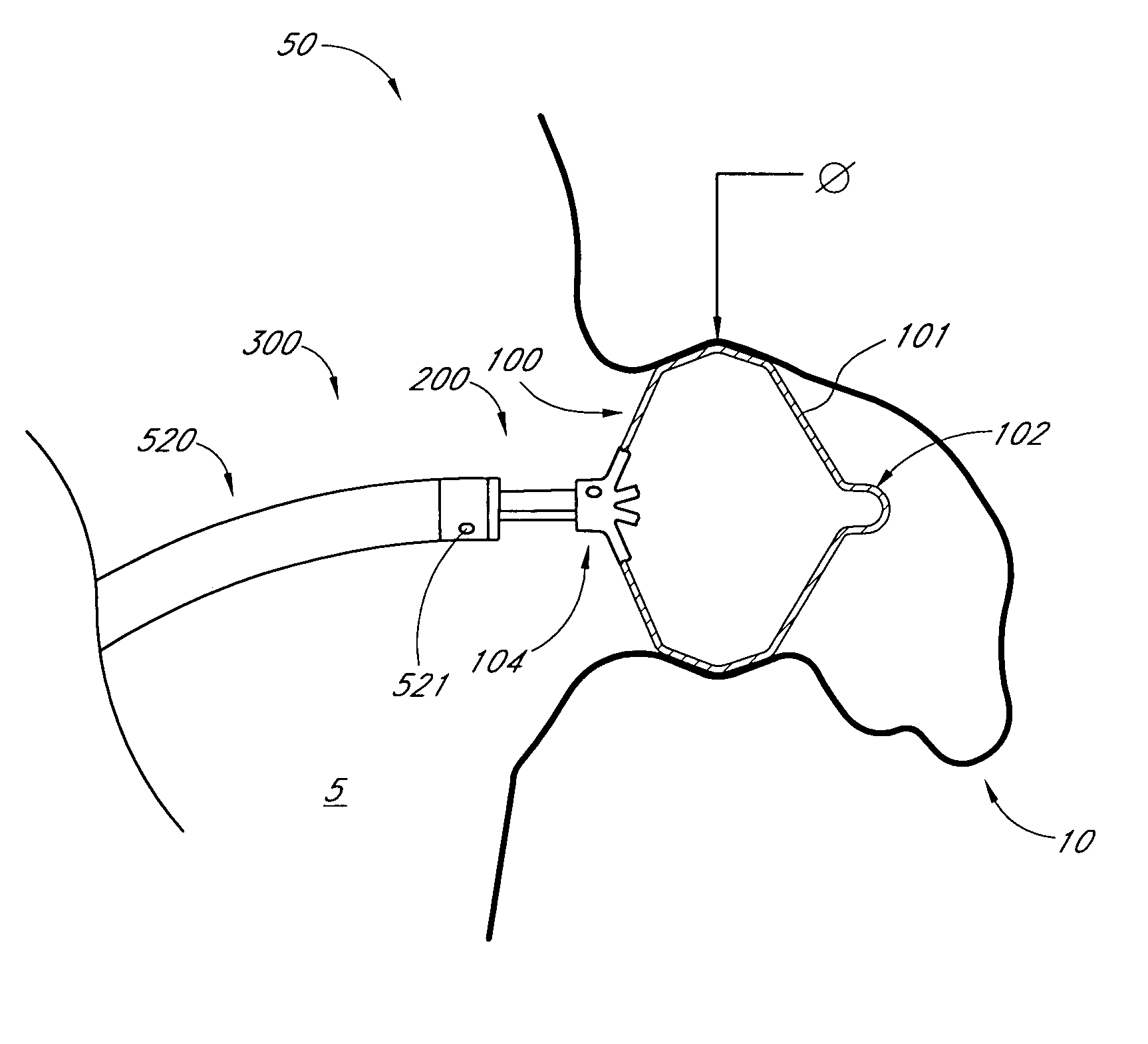
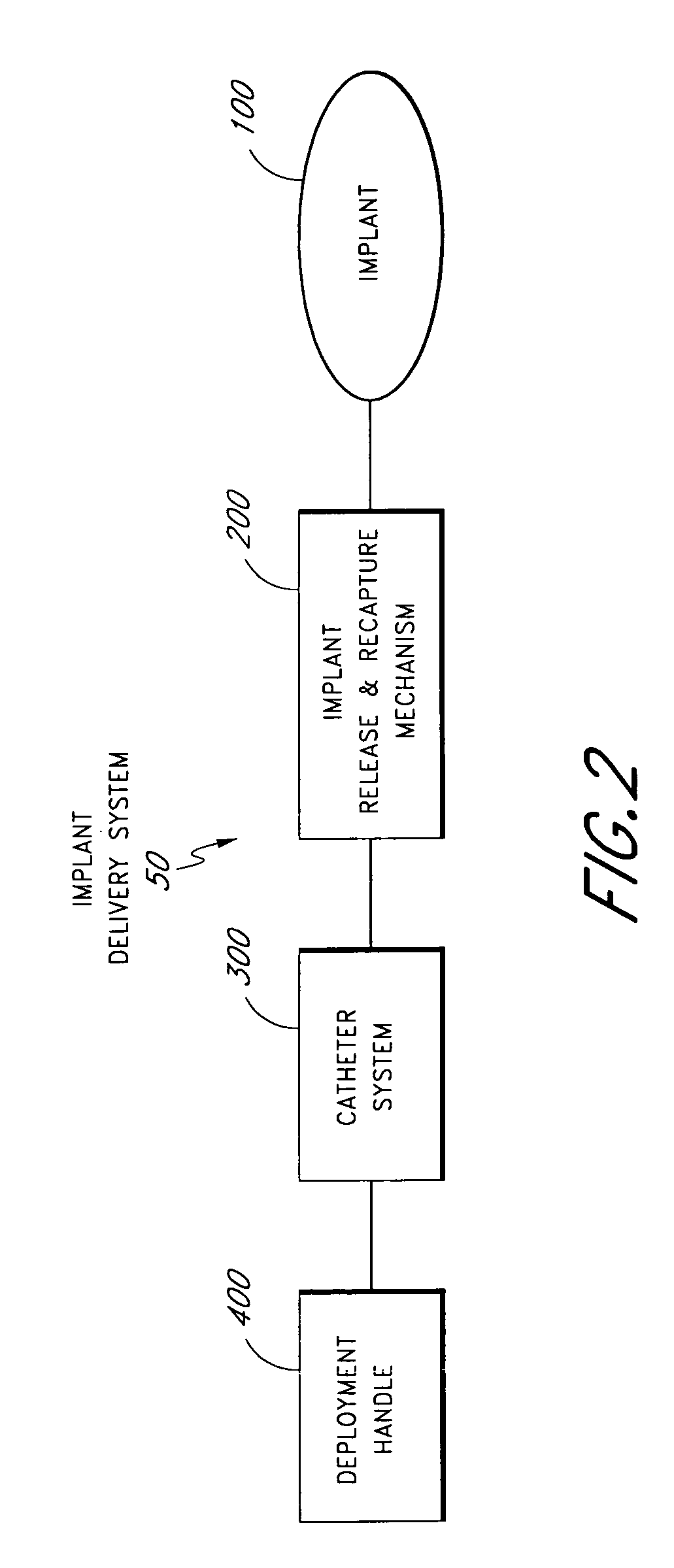
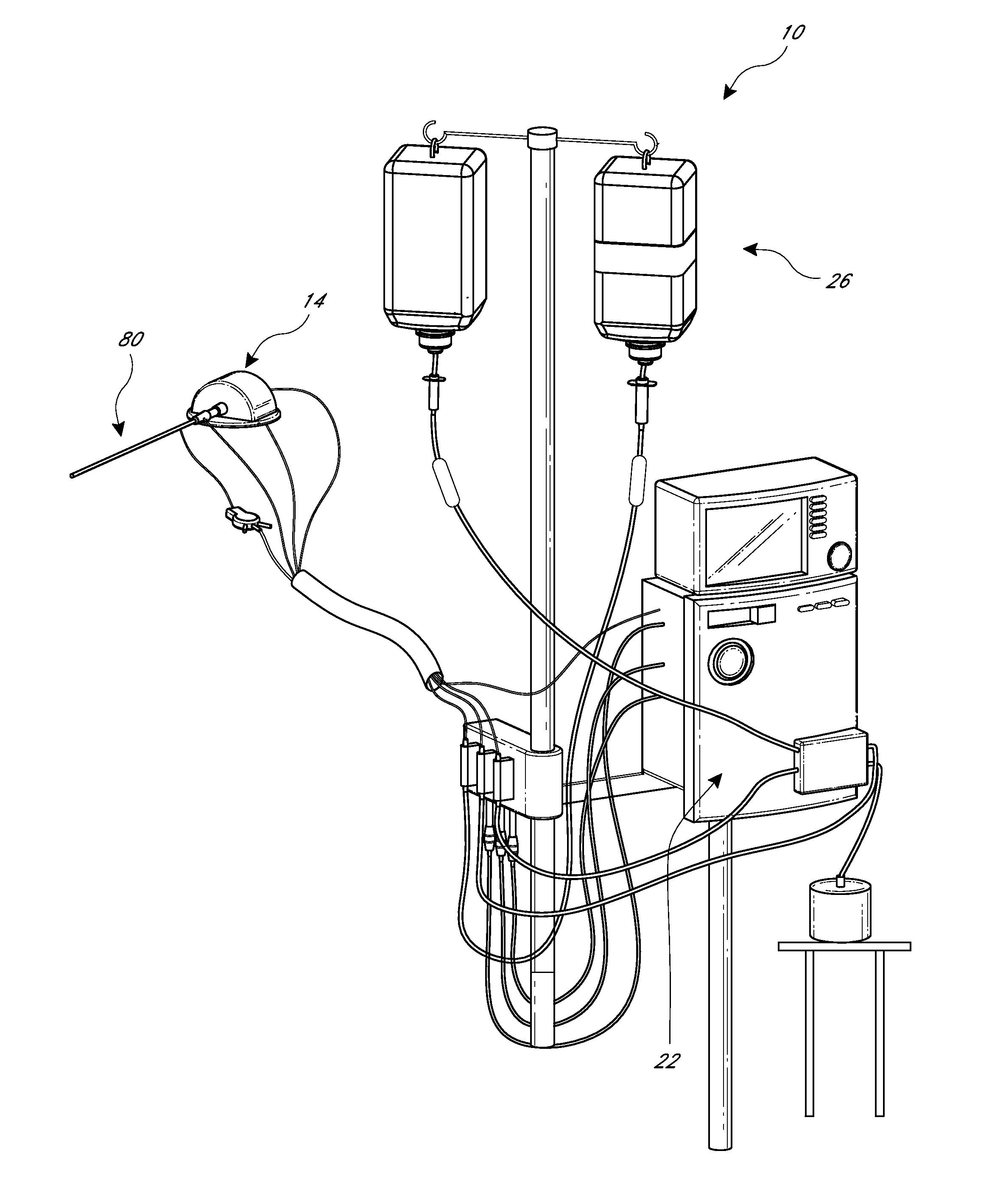
Delivery devices
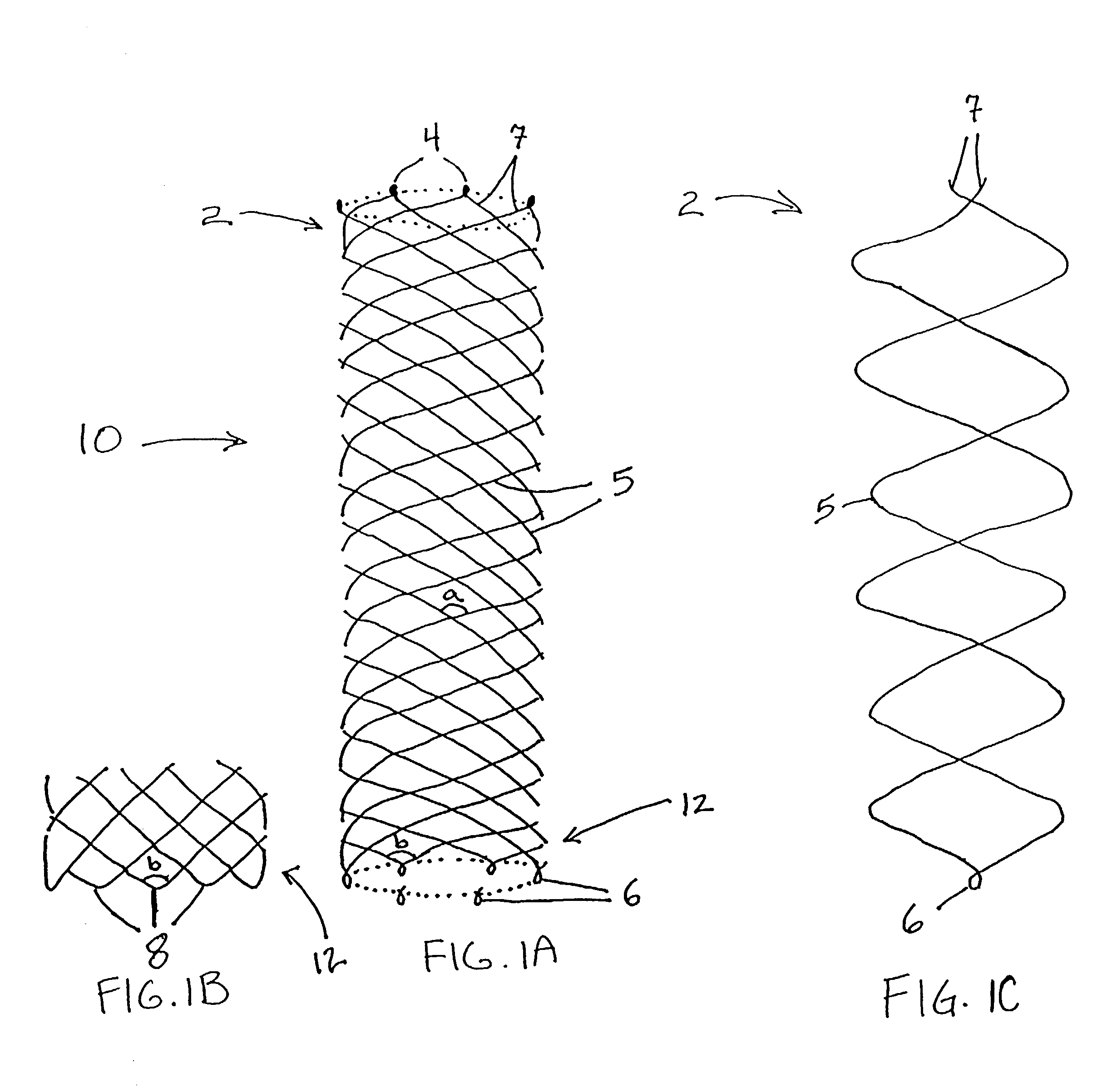
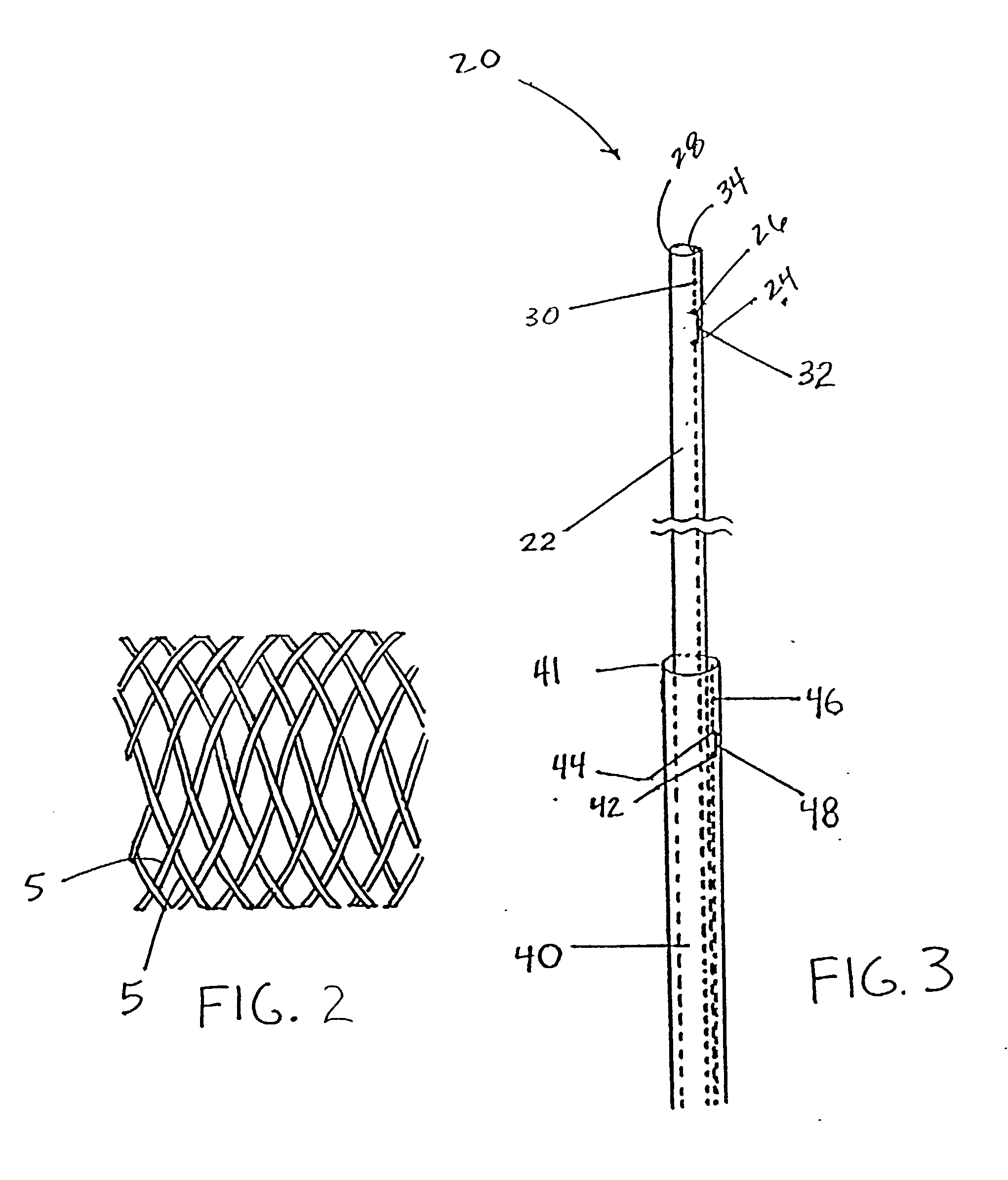
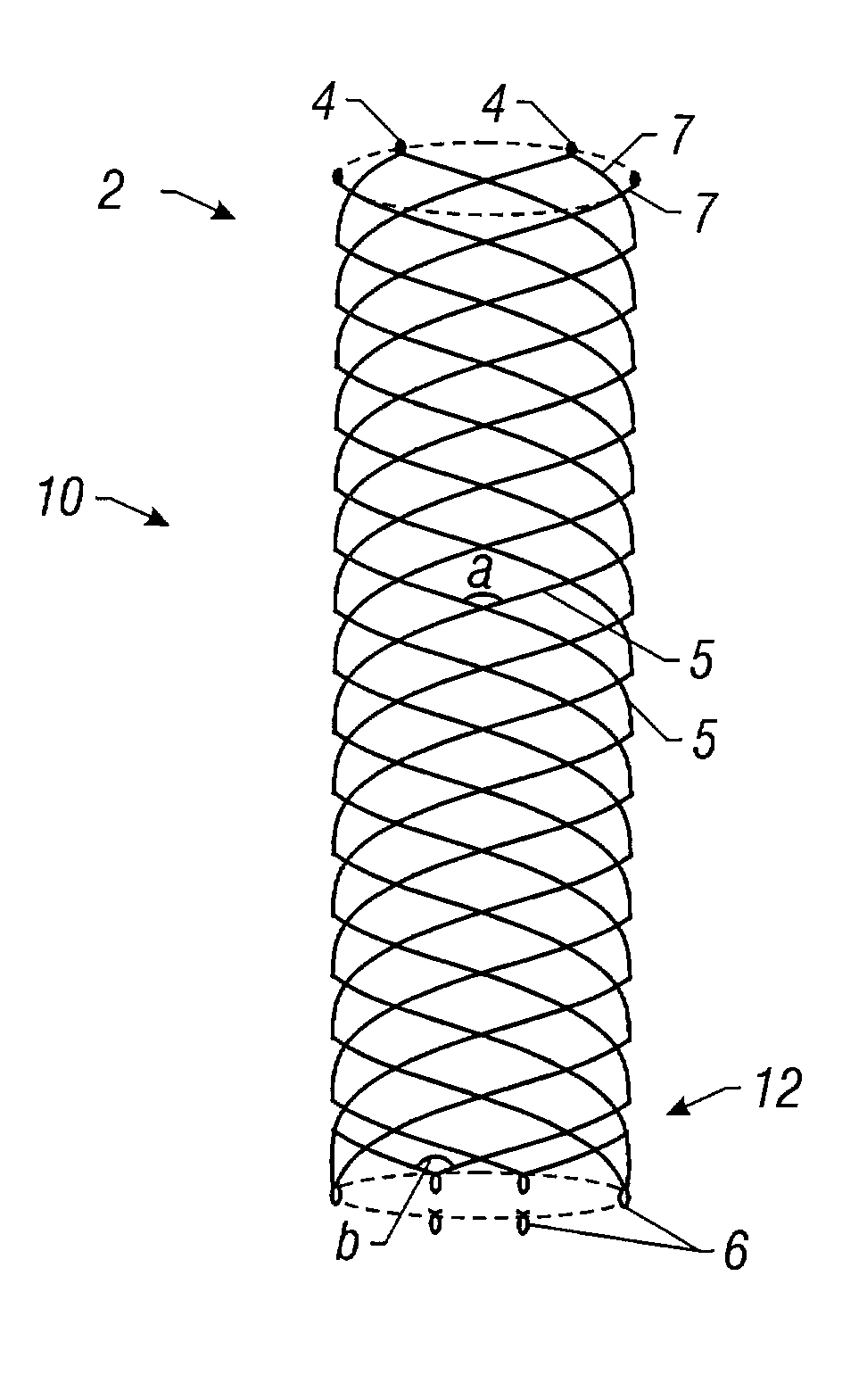
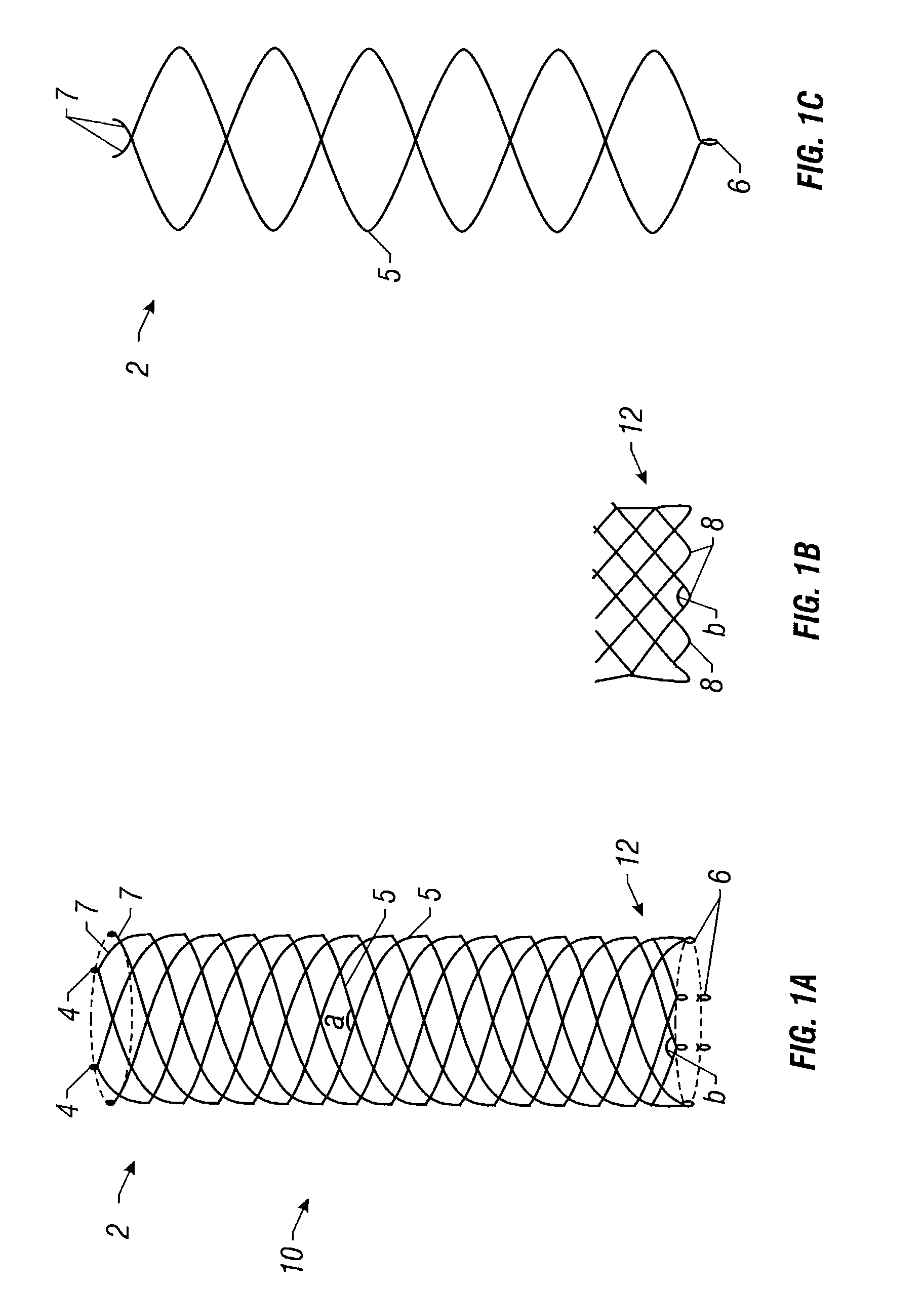
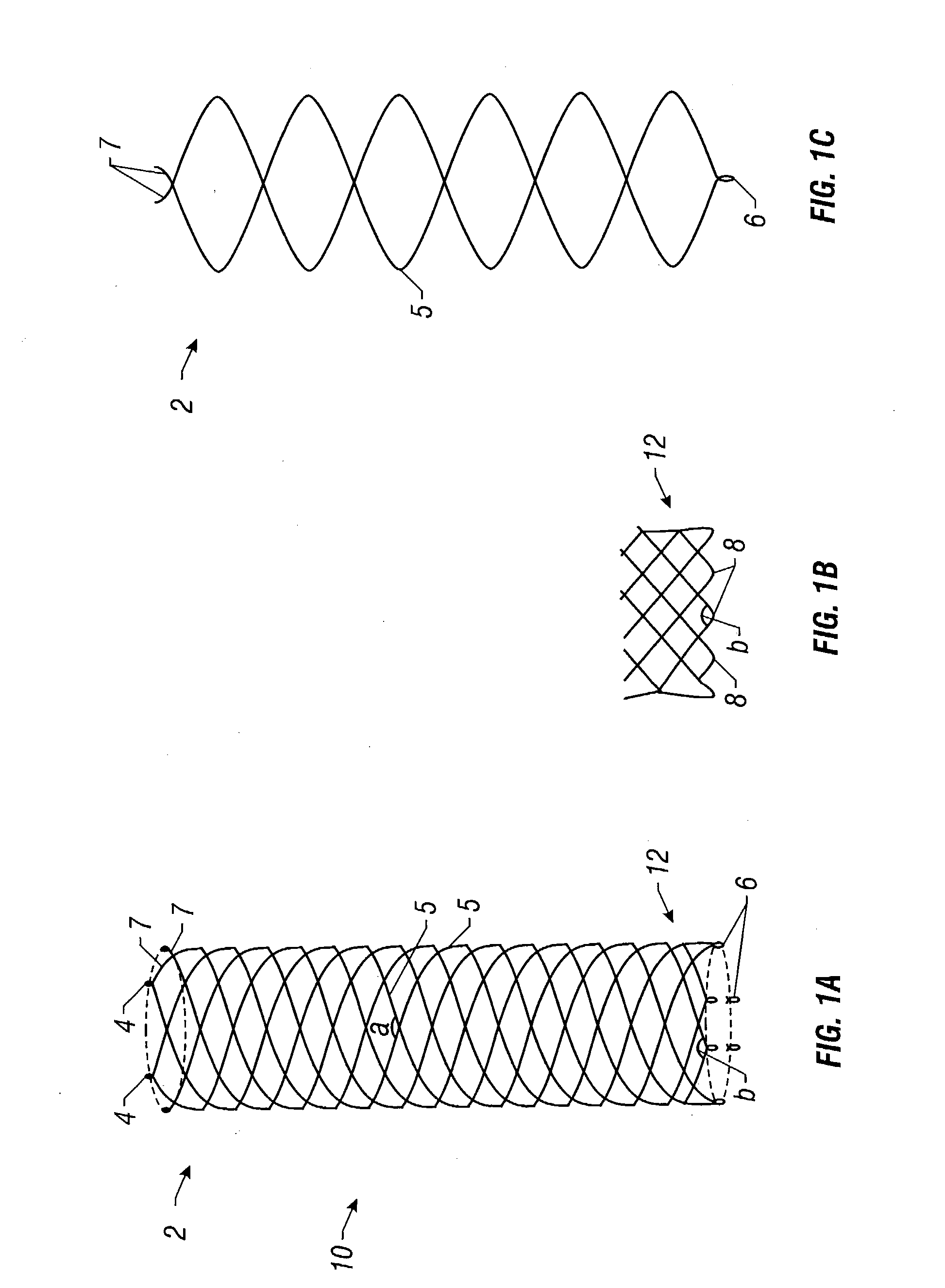
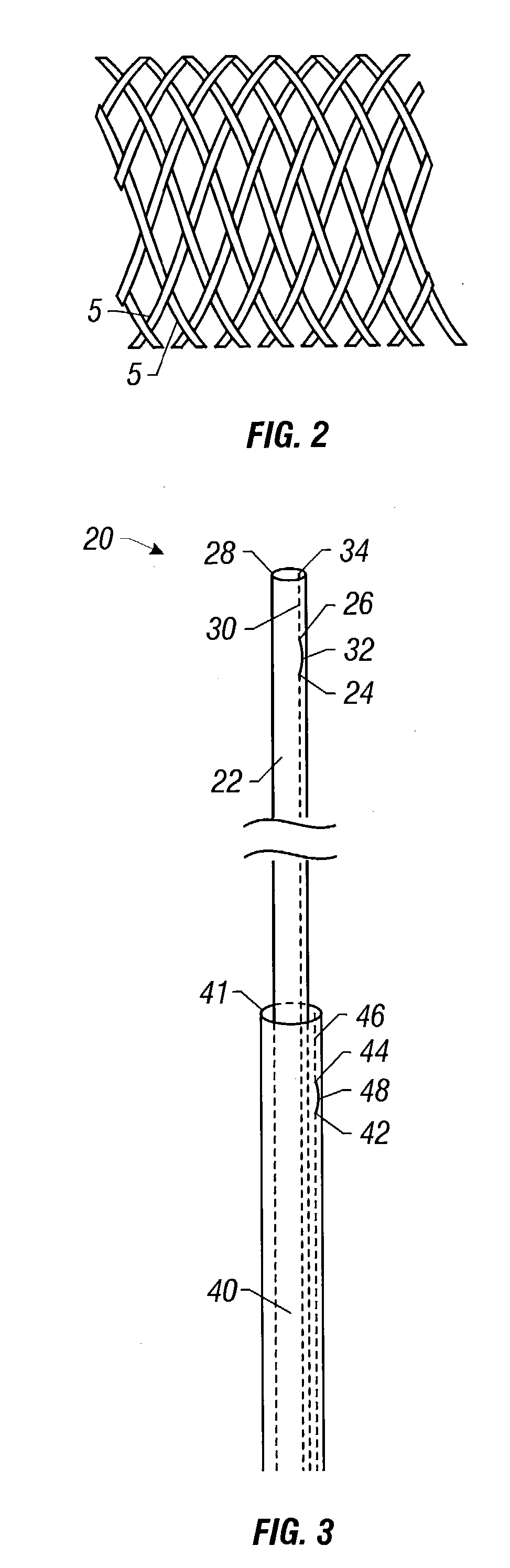
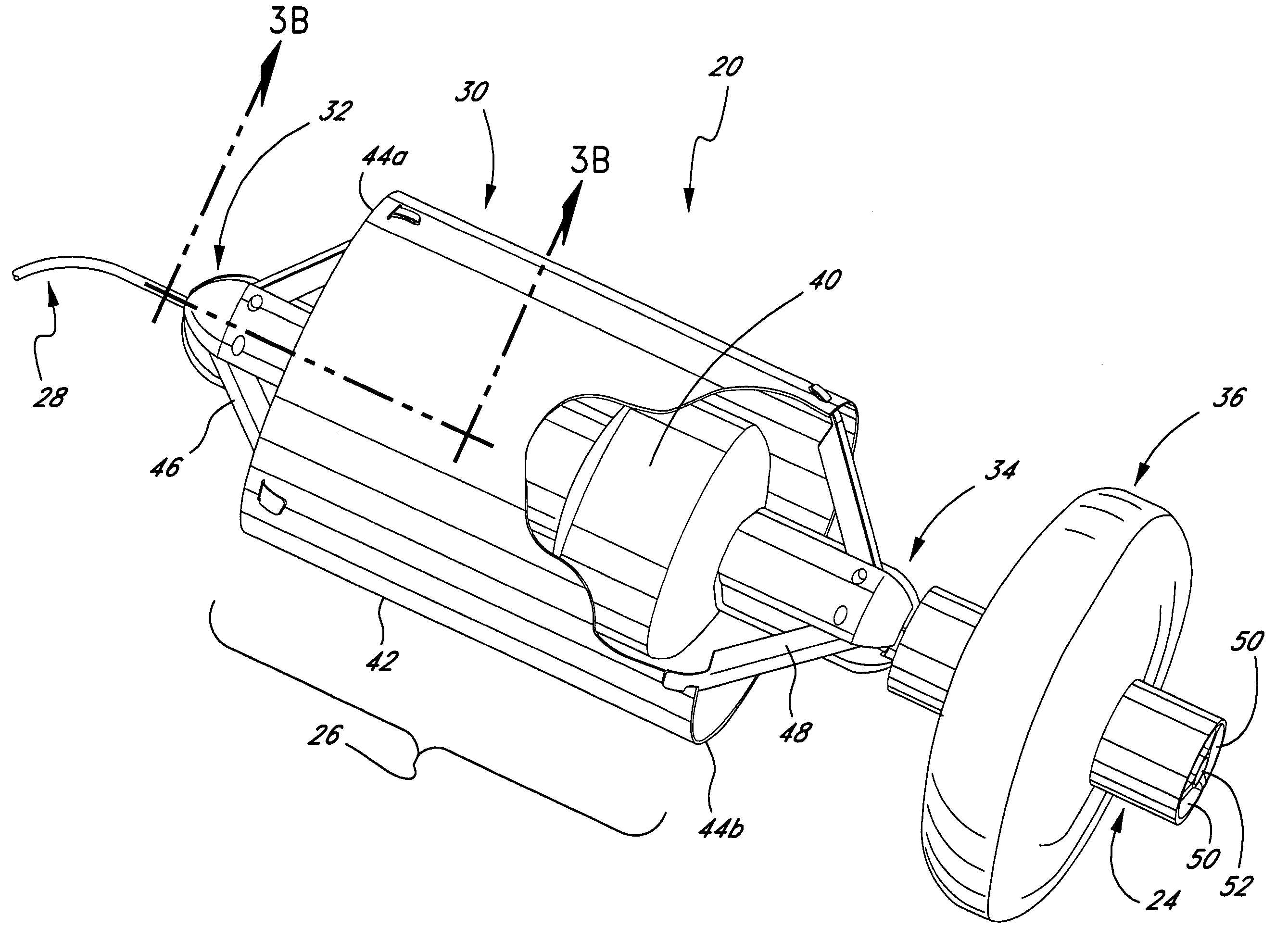
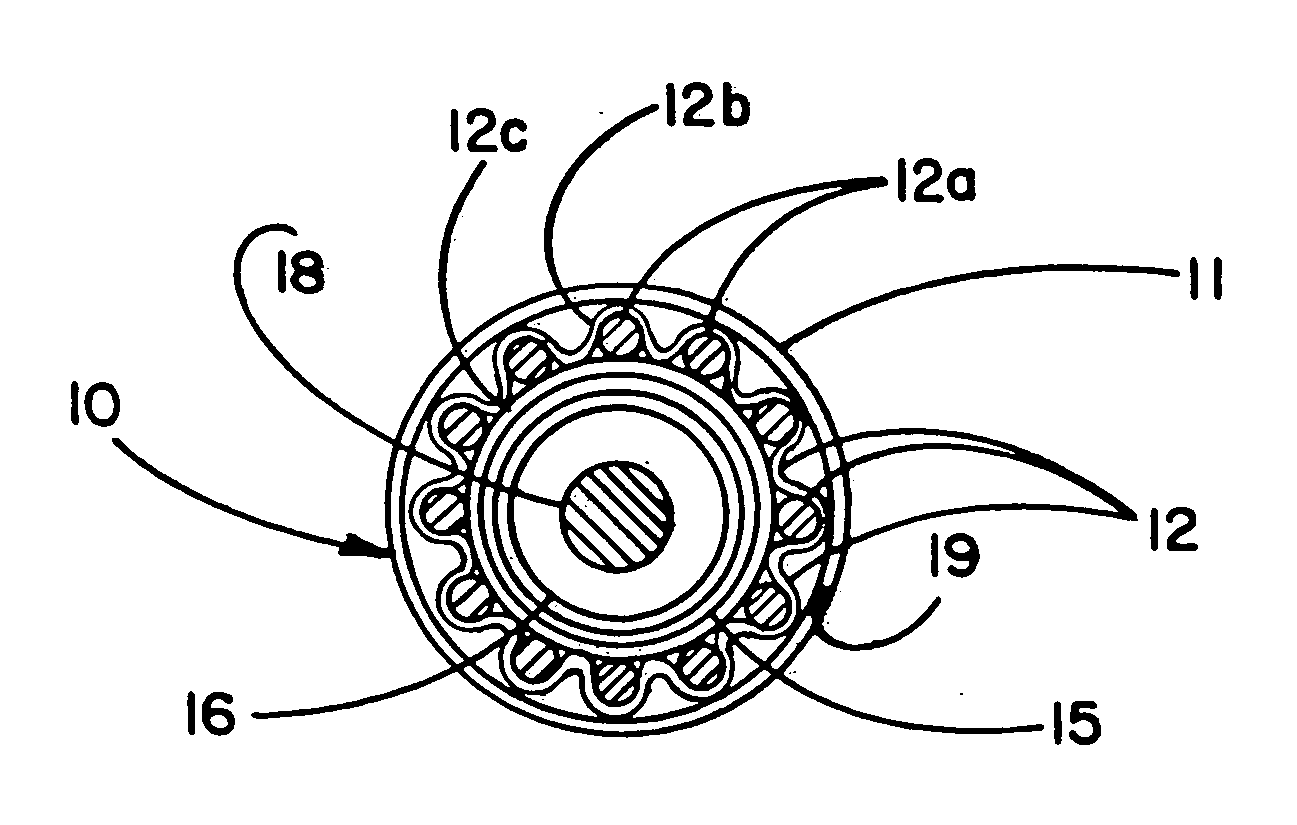
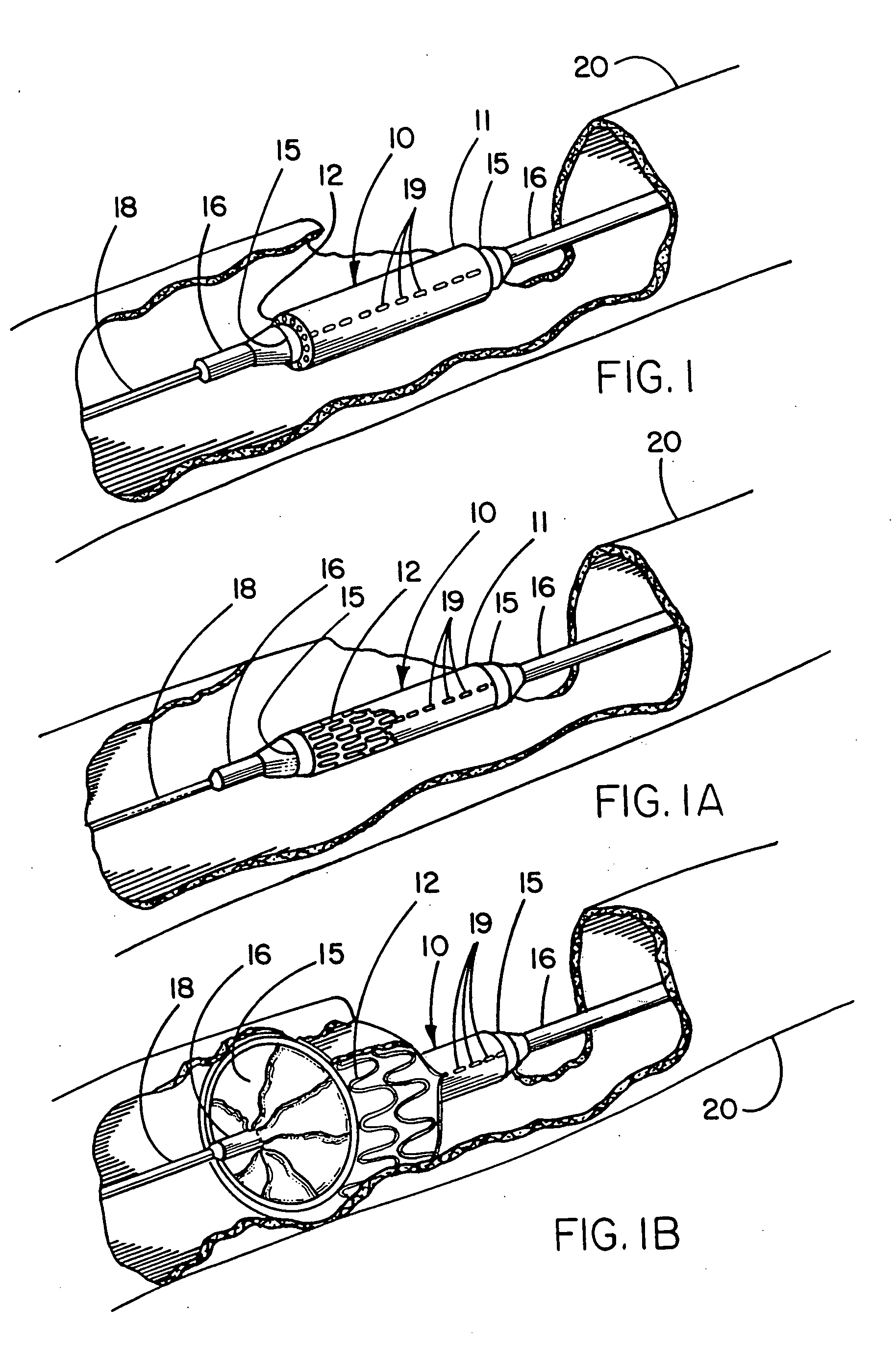
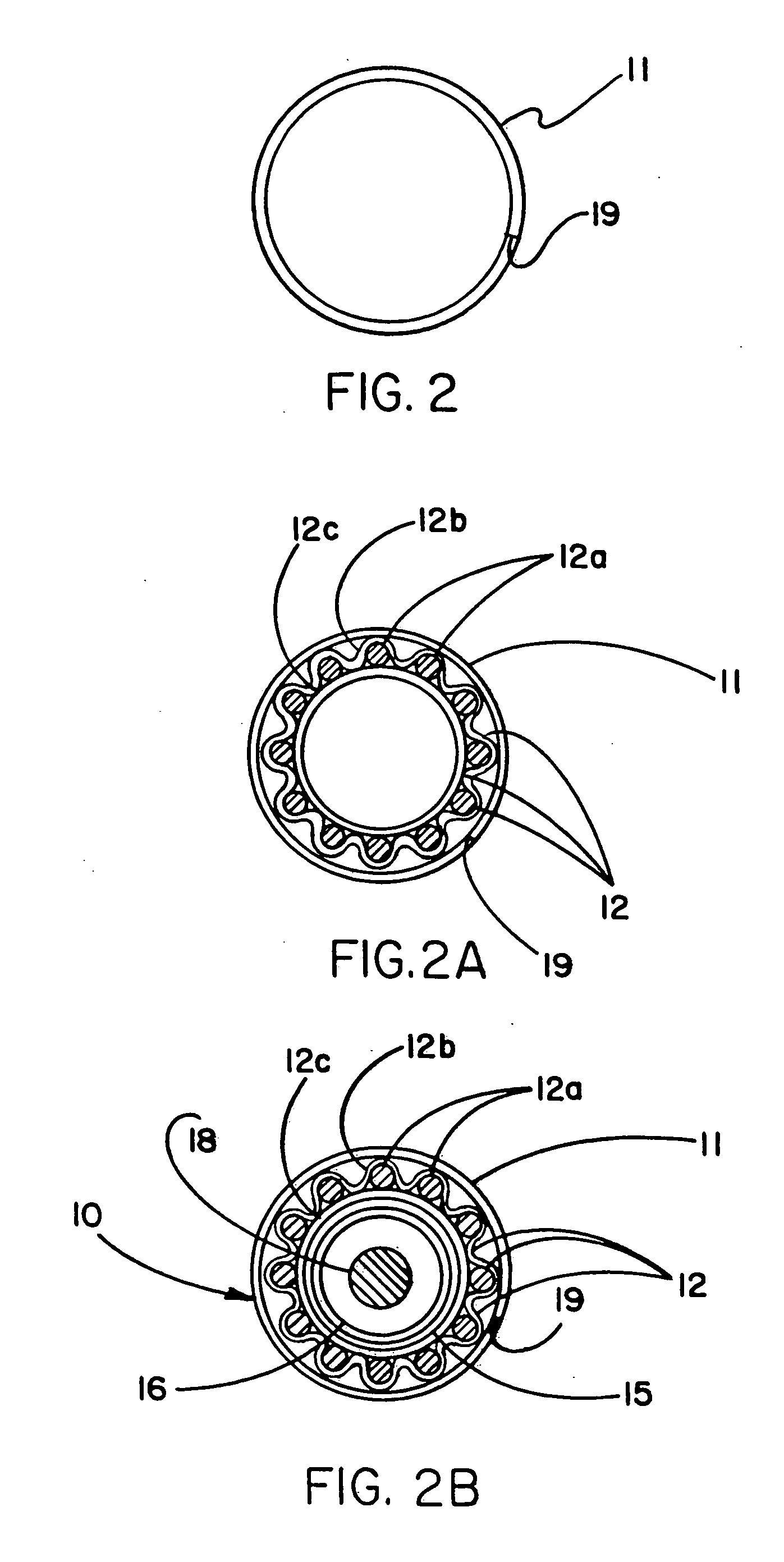
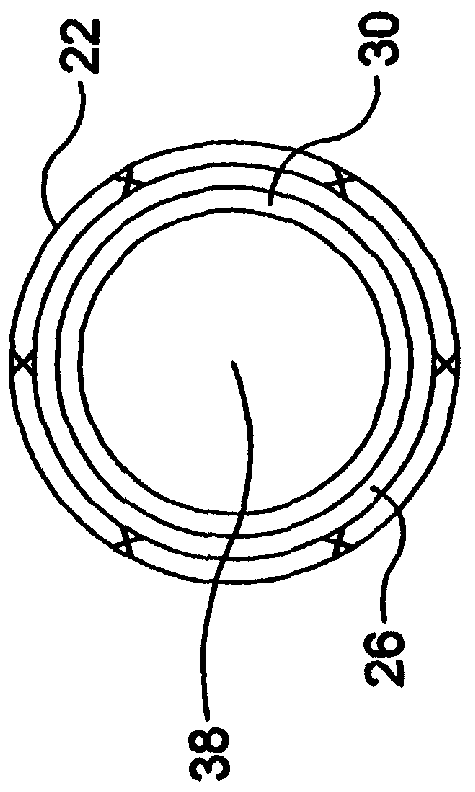
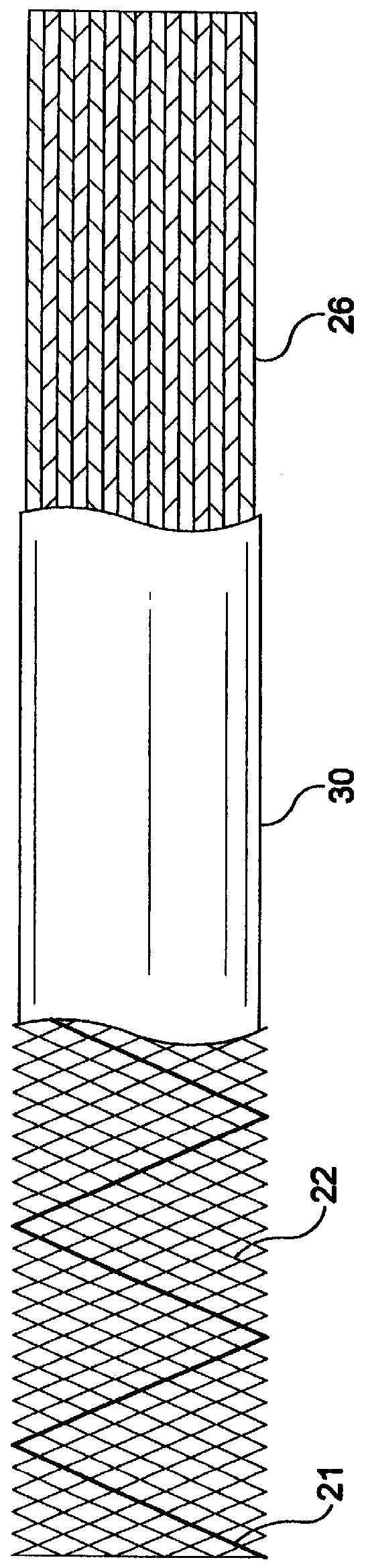
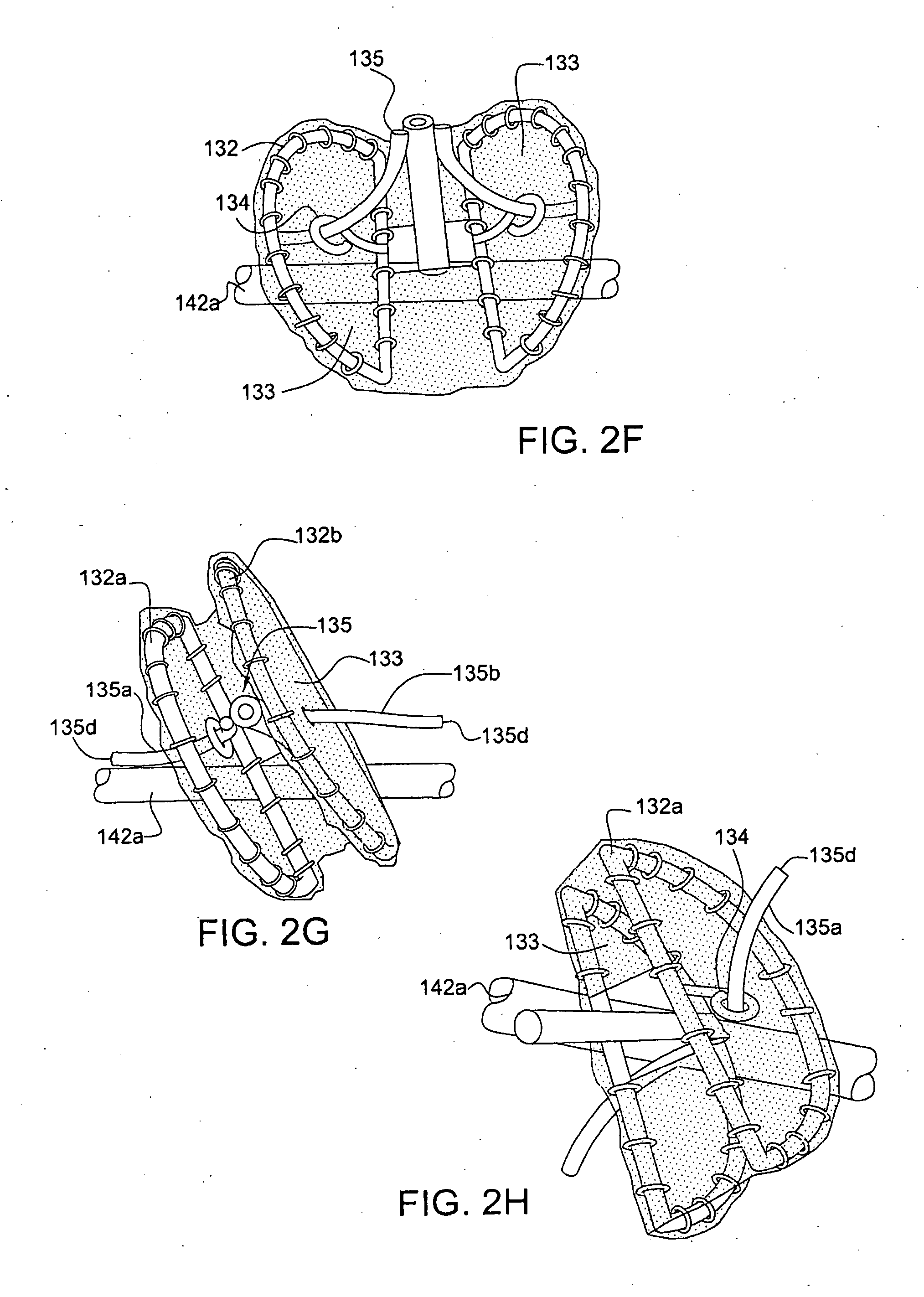
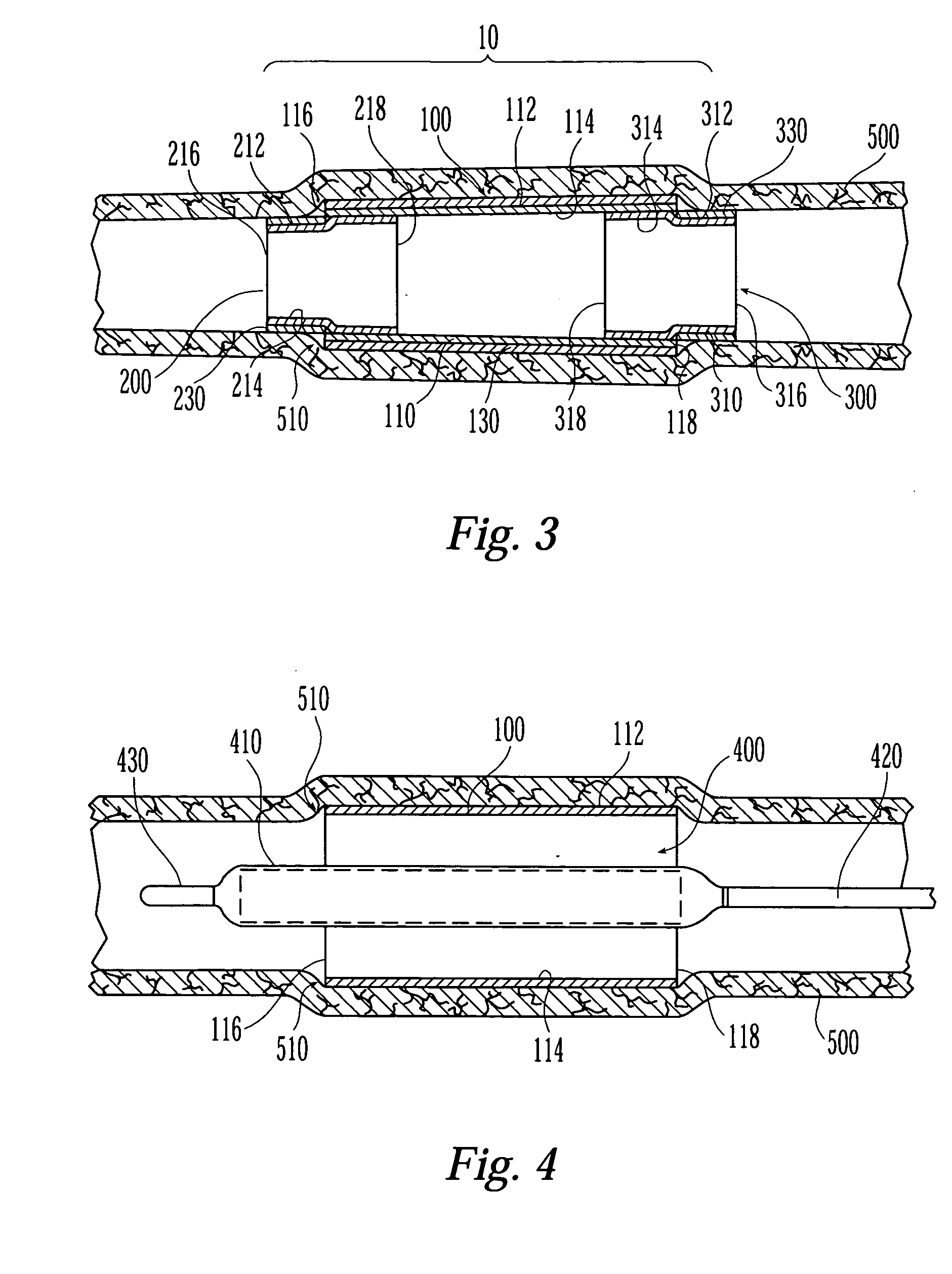
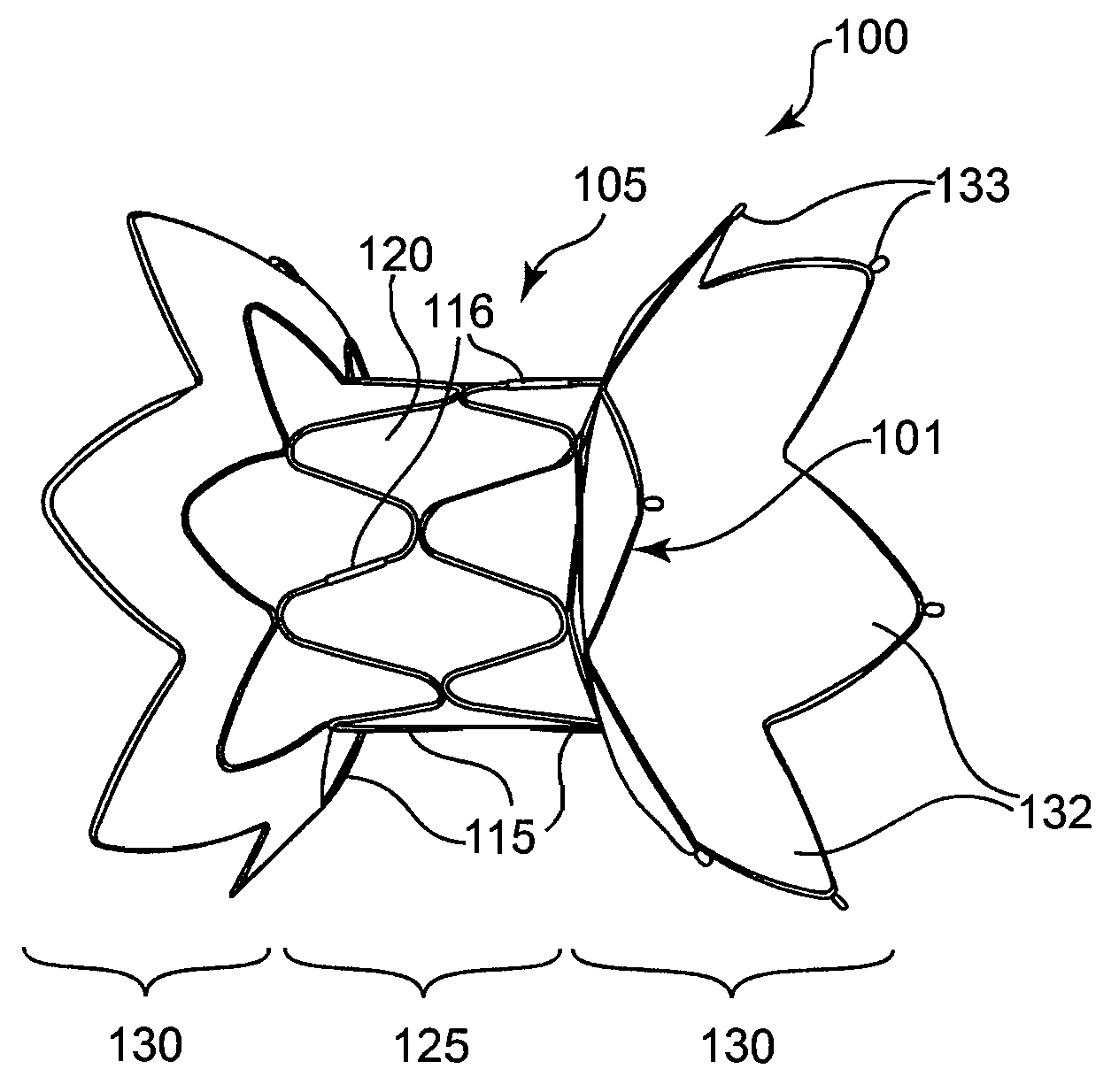
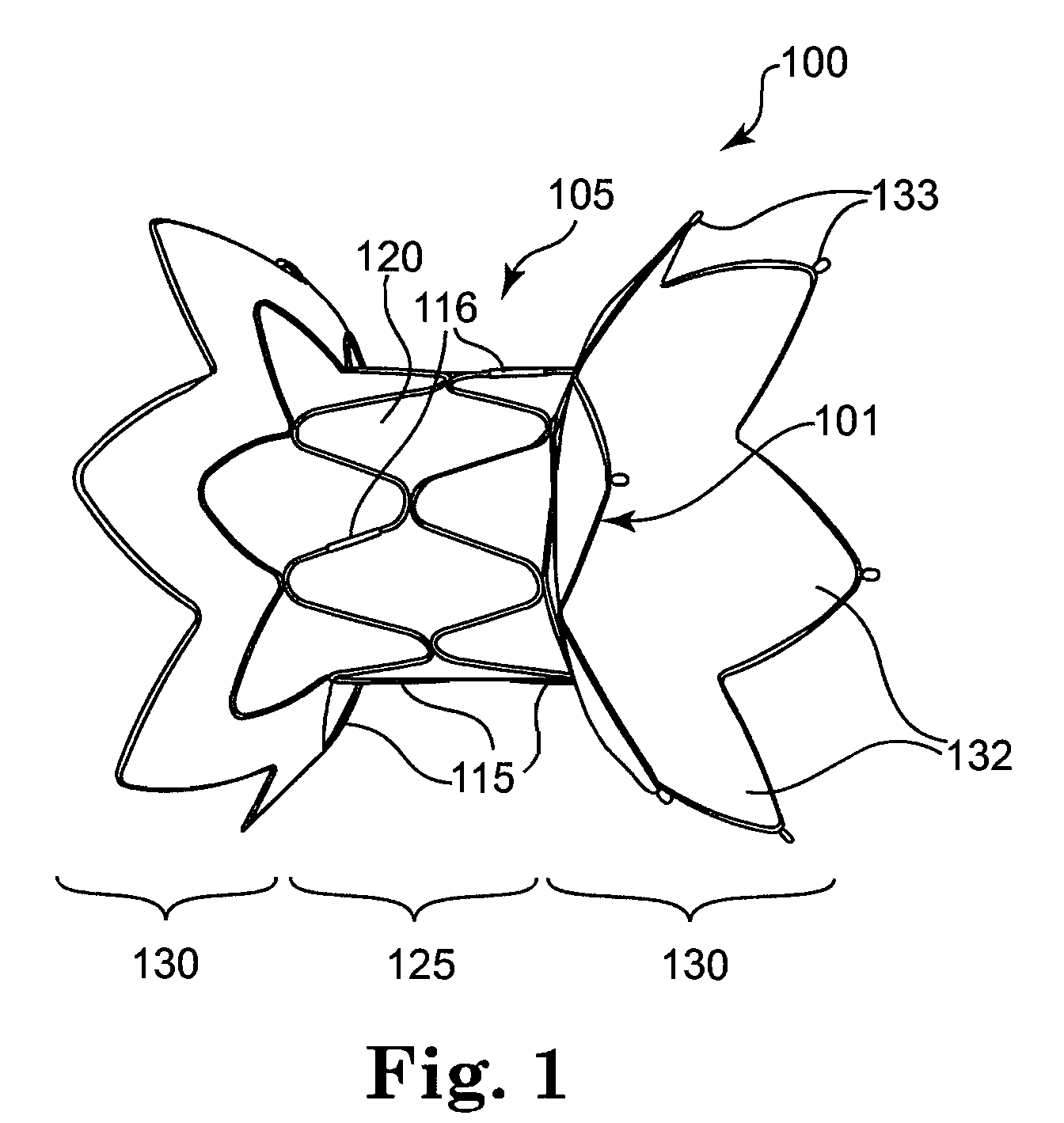
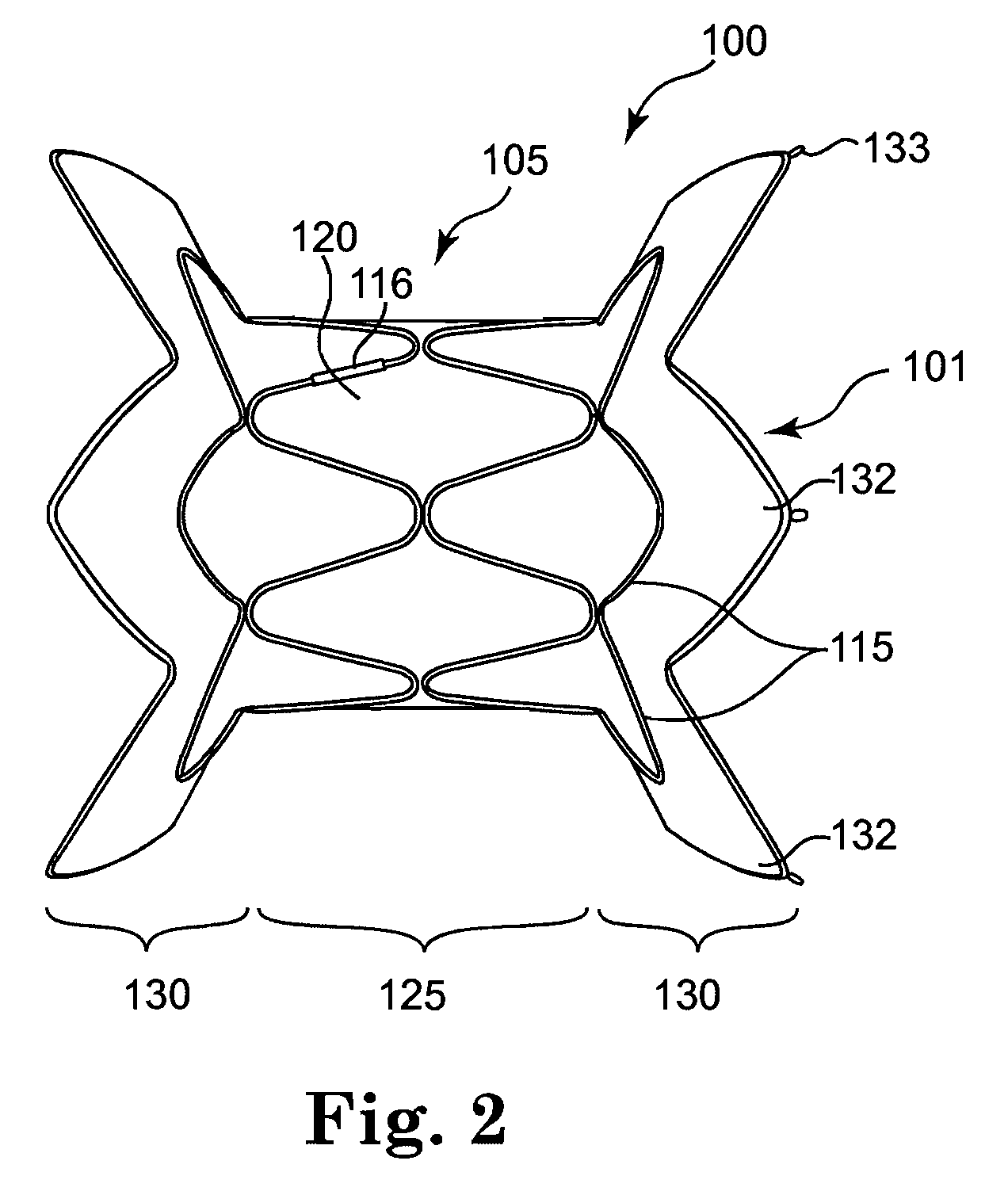
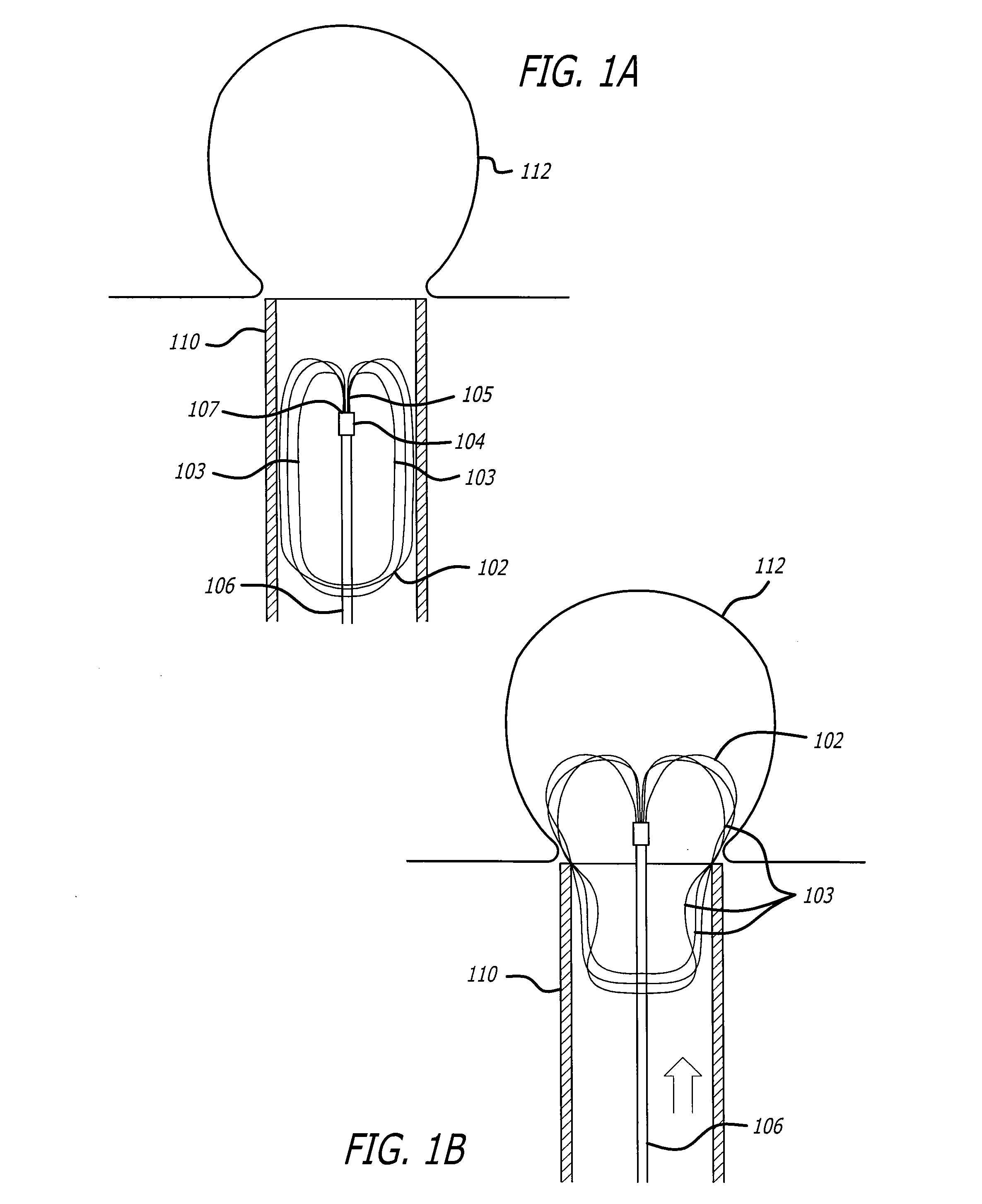
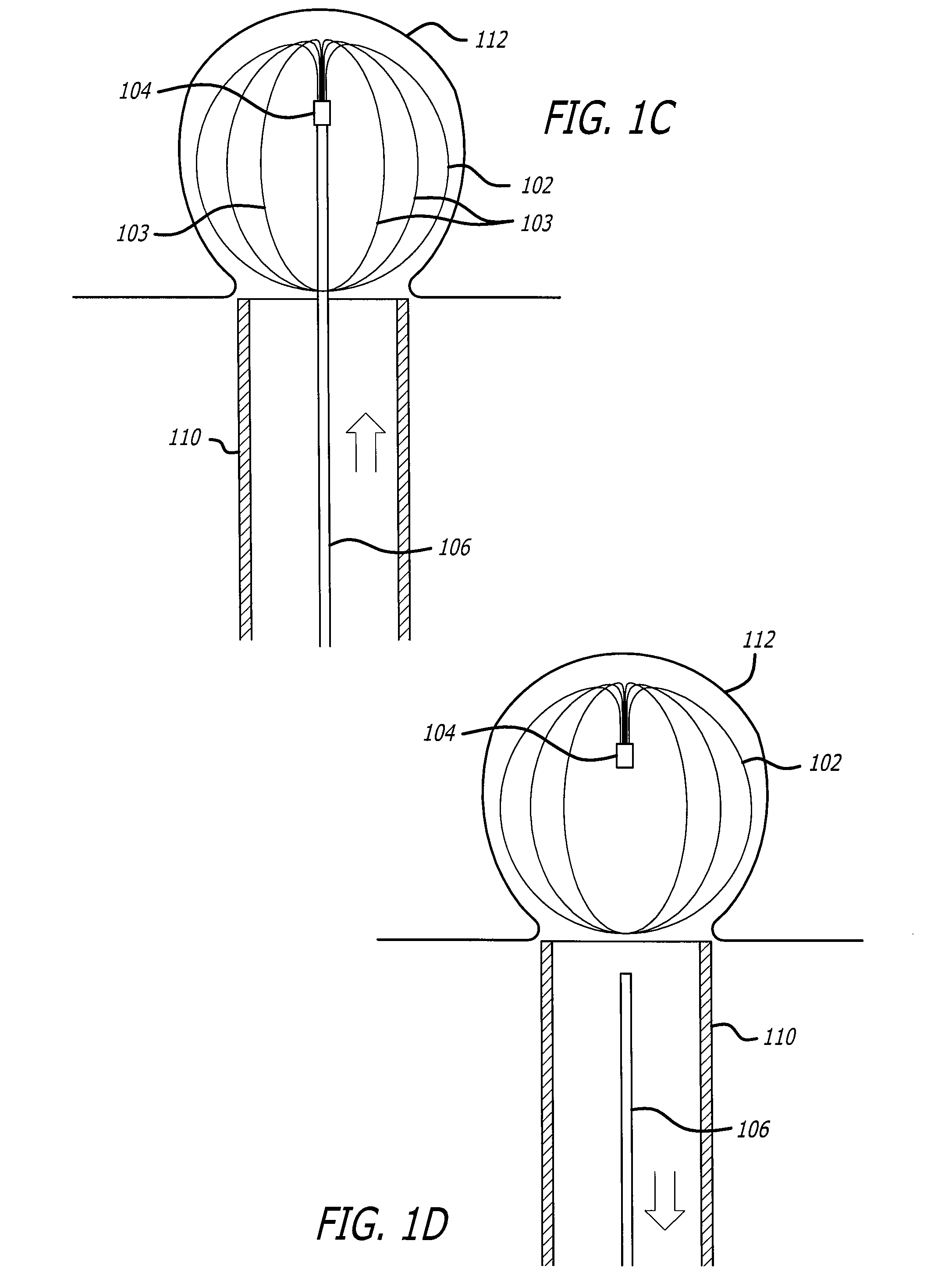
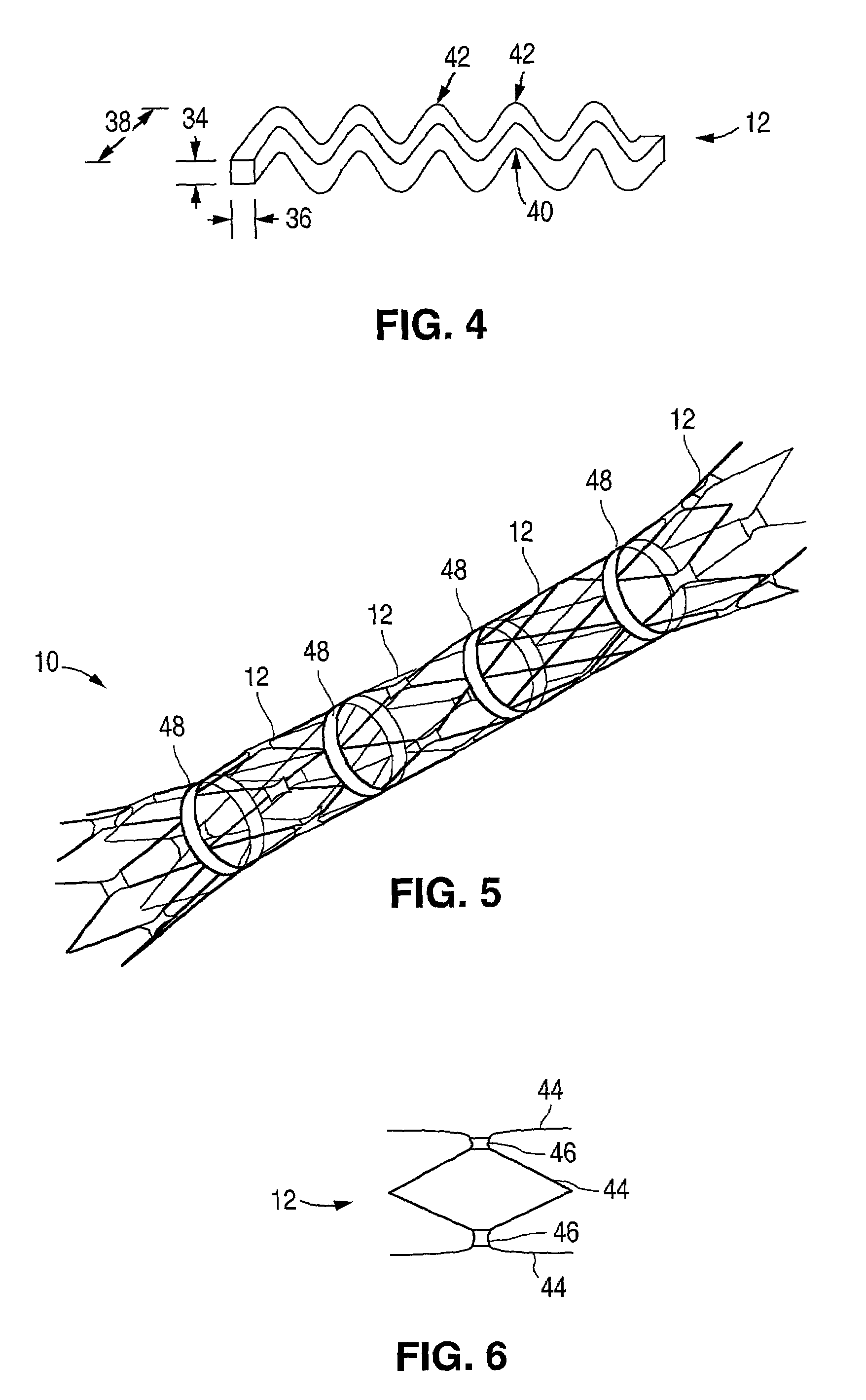
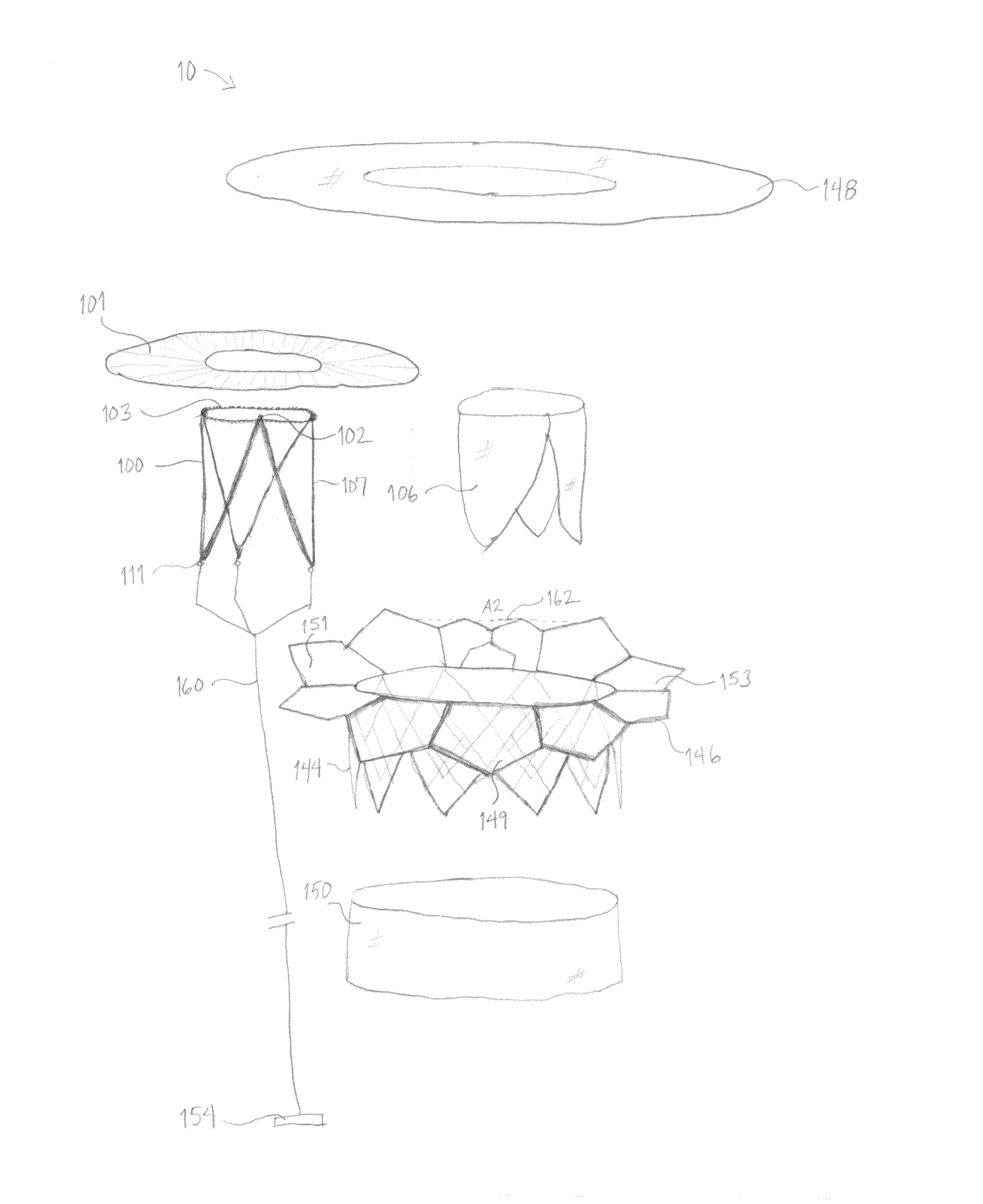
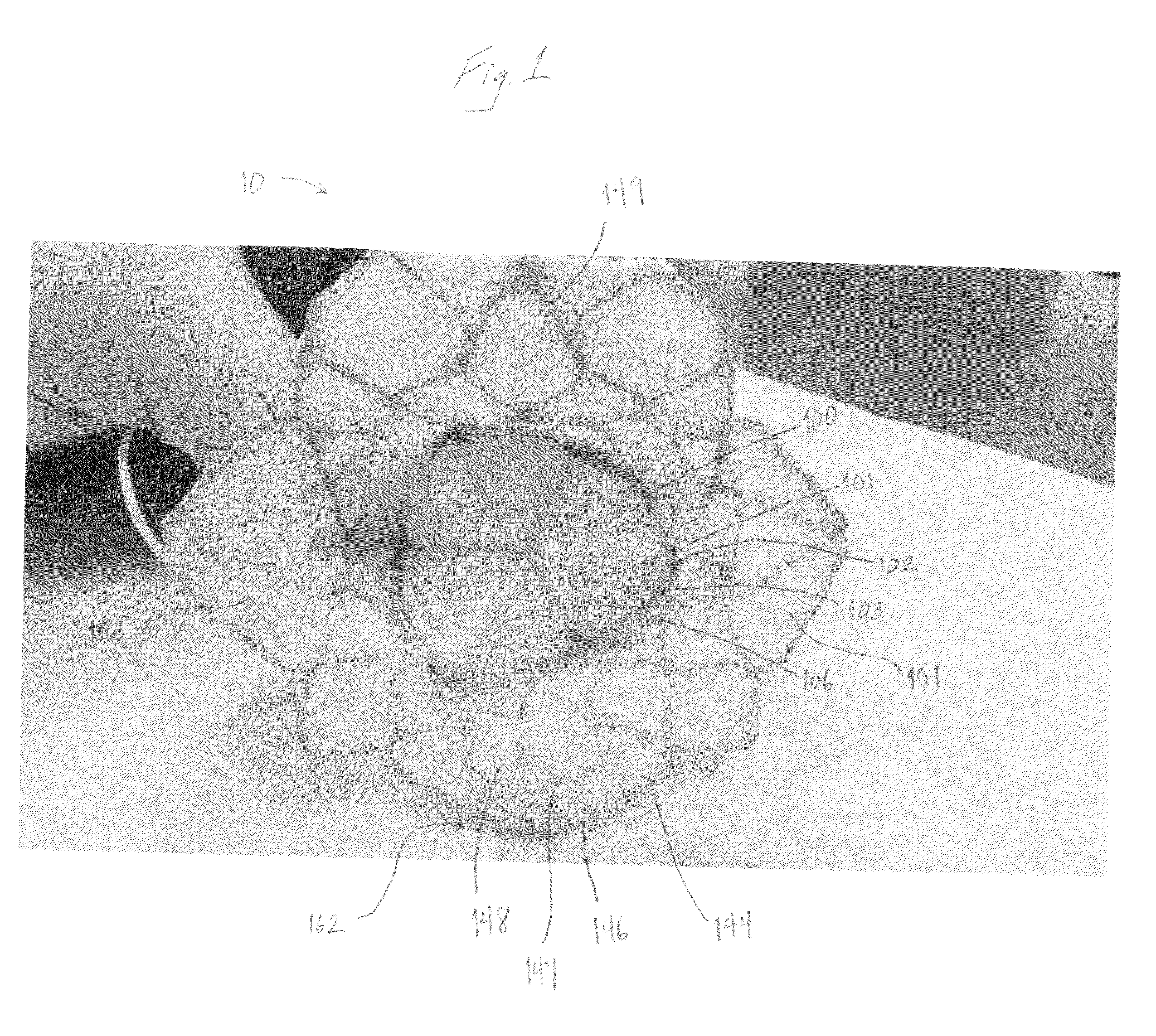
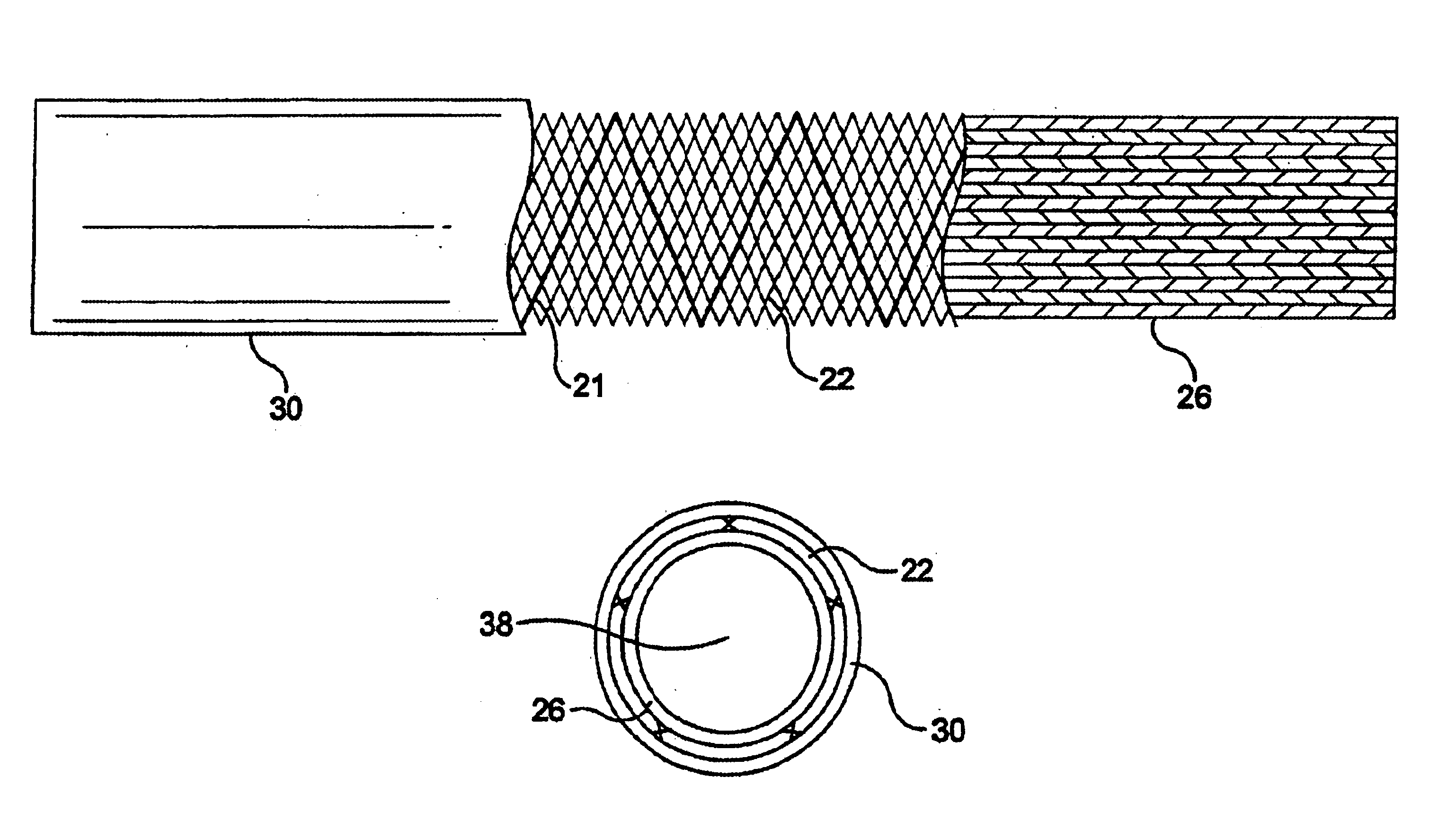
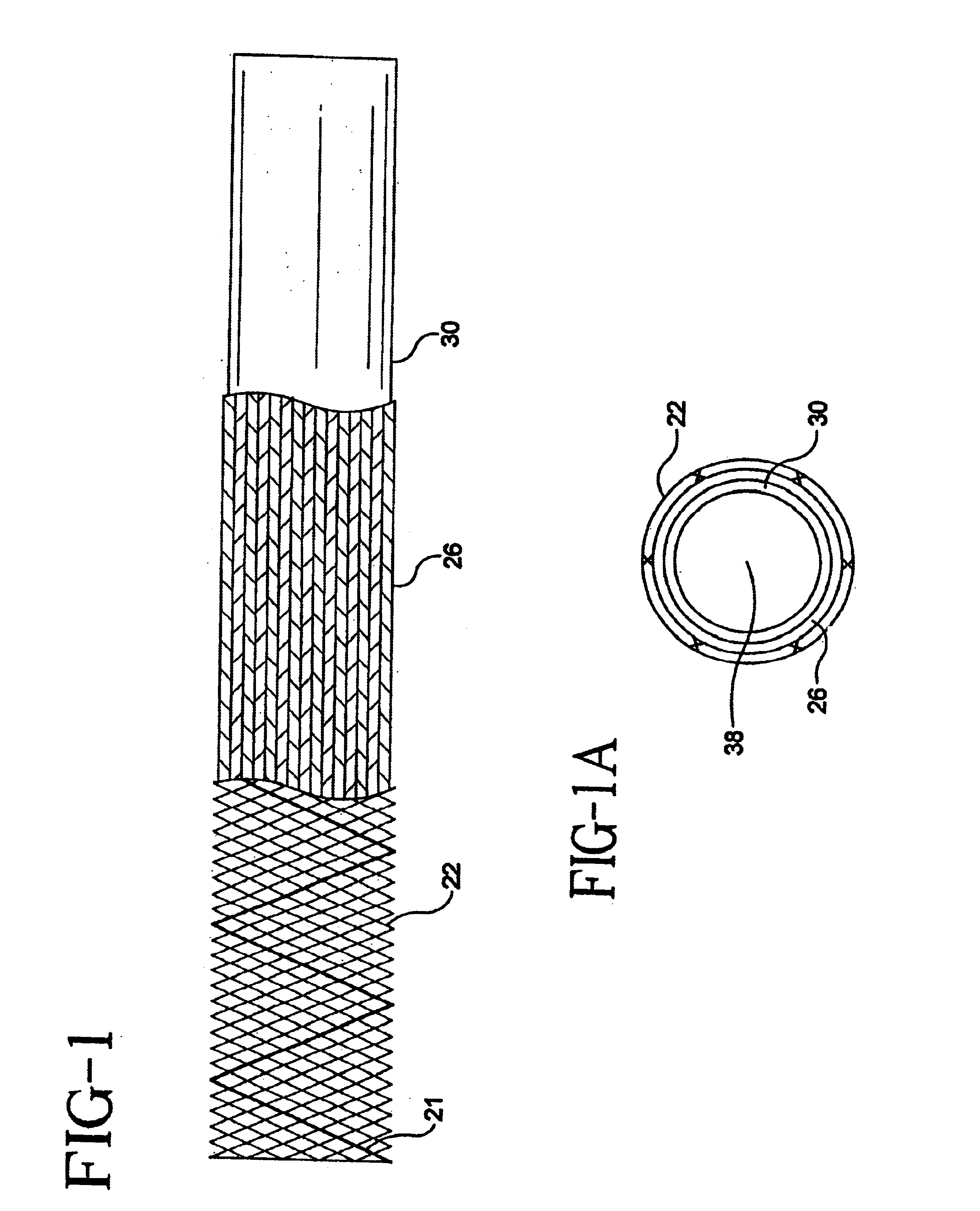
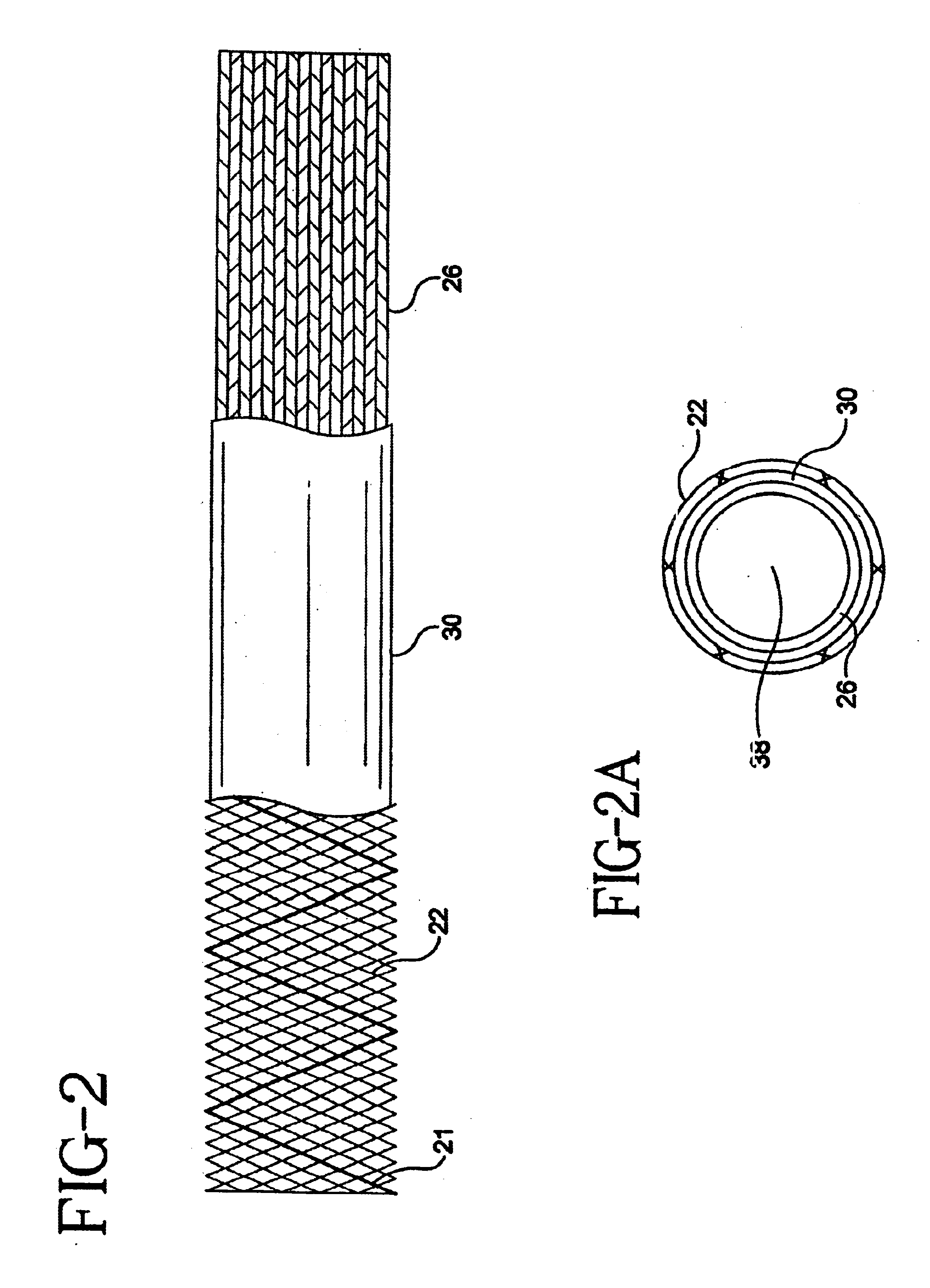
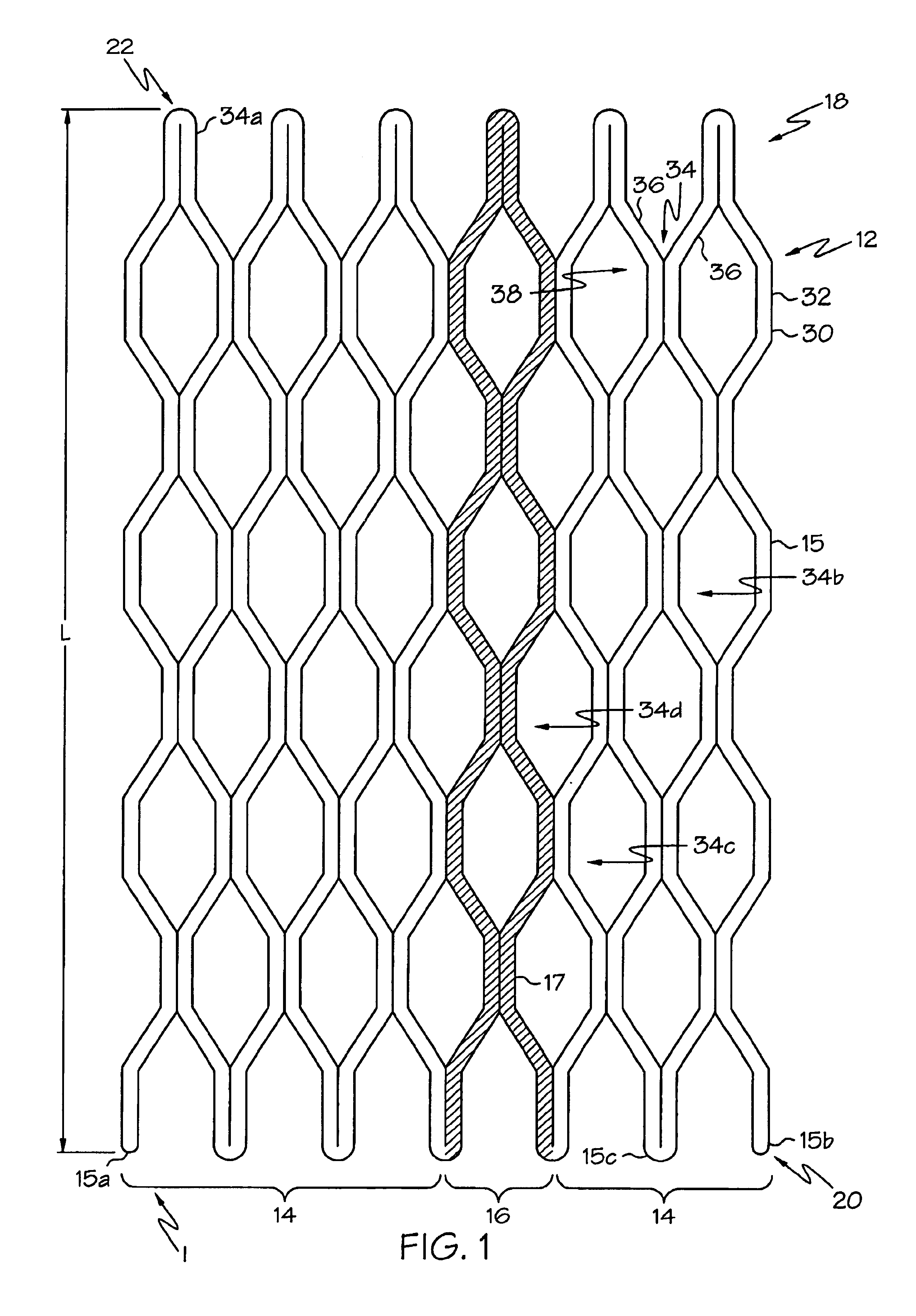
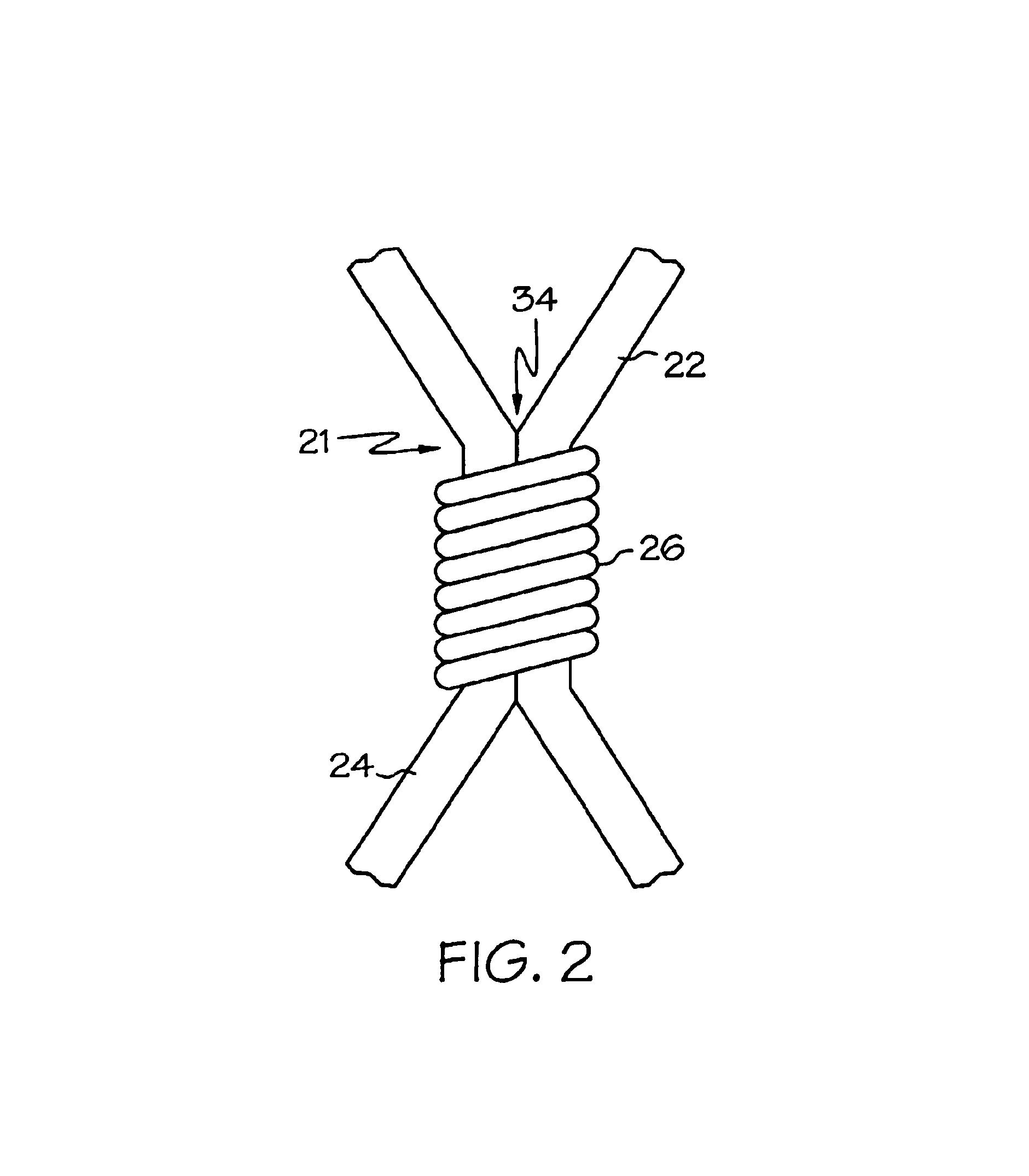
Self-expandable, woven intravascular devices for use as stents (both straight and tapered), filters (both temporary and permanent) and occluders for insertion and implantation into a variety of anatomical structures. The devices may be formed from shape memory metals such as nitinol. The devices may also be formed from biodegradable materials. Delivery systems for the devices include two hollow tubes that operate coaxially. A device is secured to the tubes prior to the implantation and delivery of the device by securing one end of the device to the outside of the inner tube and by securing the other end of the device to the outside of the outer tube. The stents may be partially or completely covered by graft materials, but may also be bare. The devices may be formed from a single wire. The devices may be formed by either hand or machine weaving. The devices may be created by bending shape memory wires around tabs projecting from a template, and weaving the ends of the wires to create the body of the device such that the wires cross each other to form a plurality of angles, at least one of the angles being obtuse. The value of the obtuse angle may be increased by axially compressing the body.
Owner:HYODOH HIDEKI +2
Methods for creating woven devices
Self-expandable, woven intravascular devices for use as stents (both straight and tapered), filters (both temporary and permanent) and occluders for insertion and implantation into a variety of anatomical structures. The devices may be formed from shape memory metals such as nitinol. The devices may also be formed from biodegradable materials. Delivery systems for the devices include two hollow tubes that operate coaxially. A device is secured to the tubes prior to the implantation and delivery of the device by securing one end of the device to the outside of the inner tube and by securing the other end of the device to the outside of the outer tube. The stents may be partially or completely covered by graft materials, but may also be bare. The devices may be formed from a single wire. The devices may be formed by either hand or machine weaving. The devices may be created by bending shape memory wires around tabs projecting from a template, and weaving the ends of the wires to create the body of the device such that the wires cross each other to form a plurality of angles, at least one of the angles being obtuse. The value of the obtuse angle may be increased by axially compressing the body.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
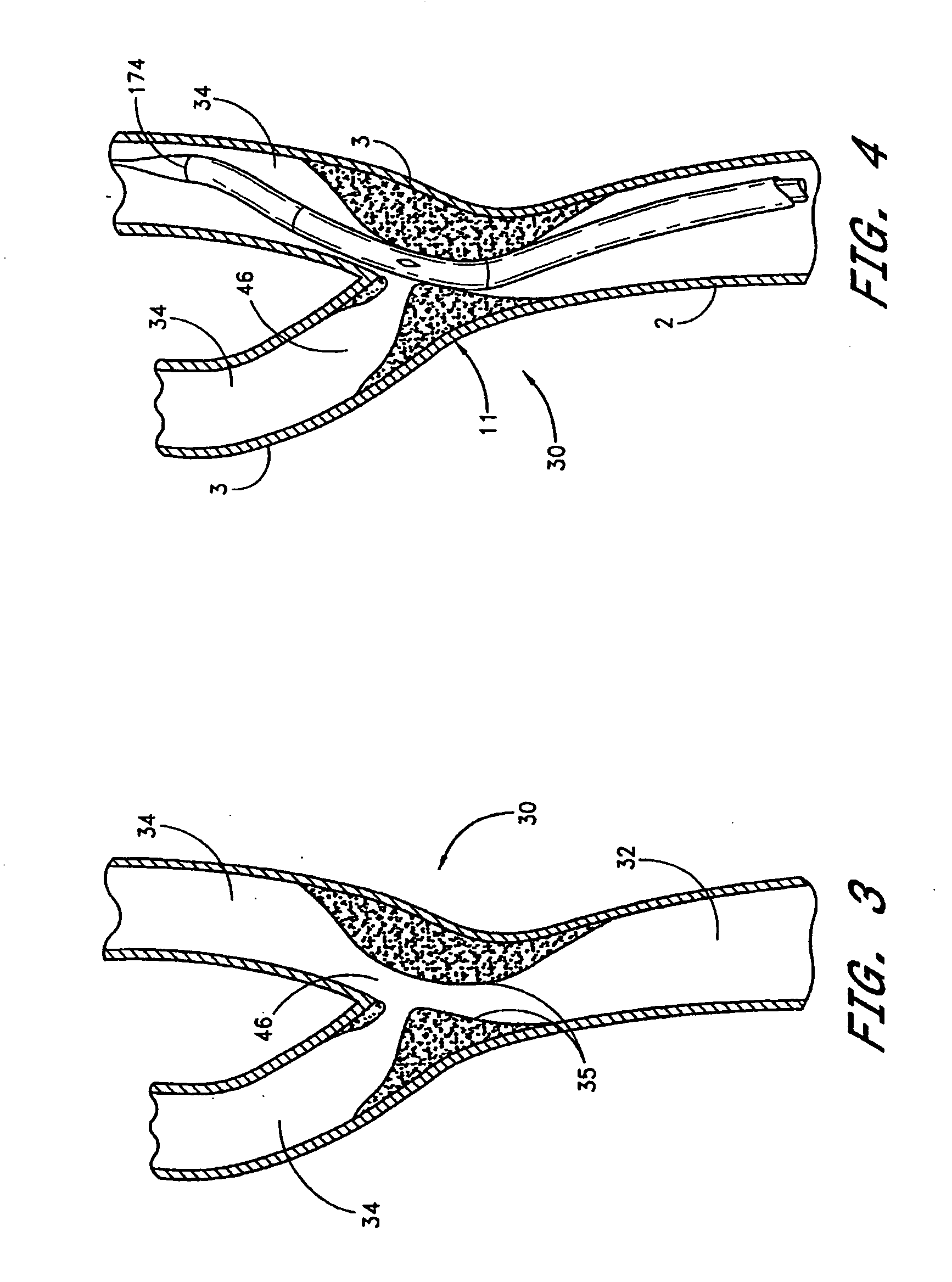
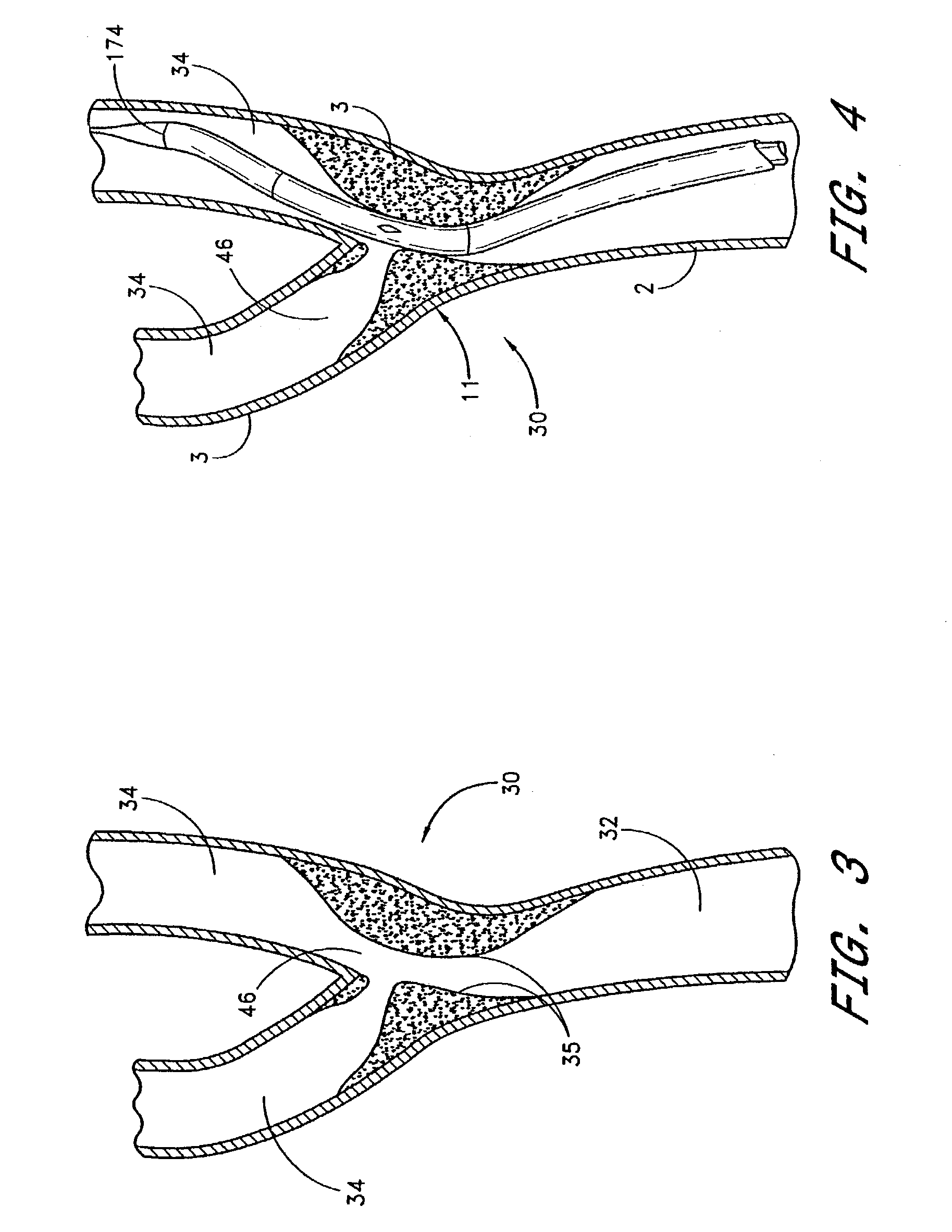
Noncylindrical stent deployment system for treating vascular bifurcations
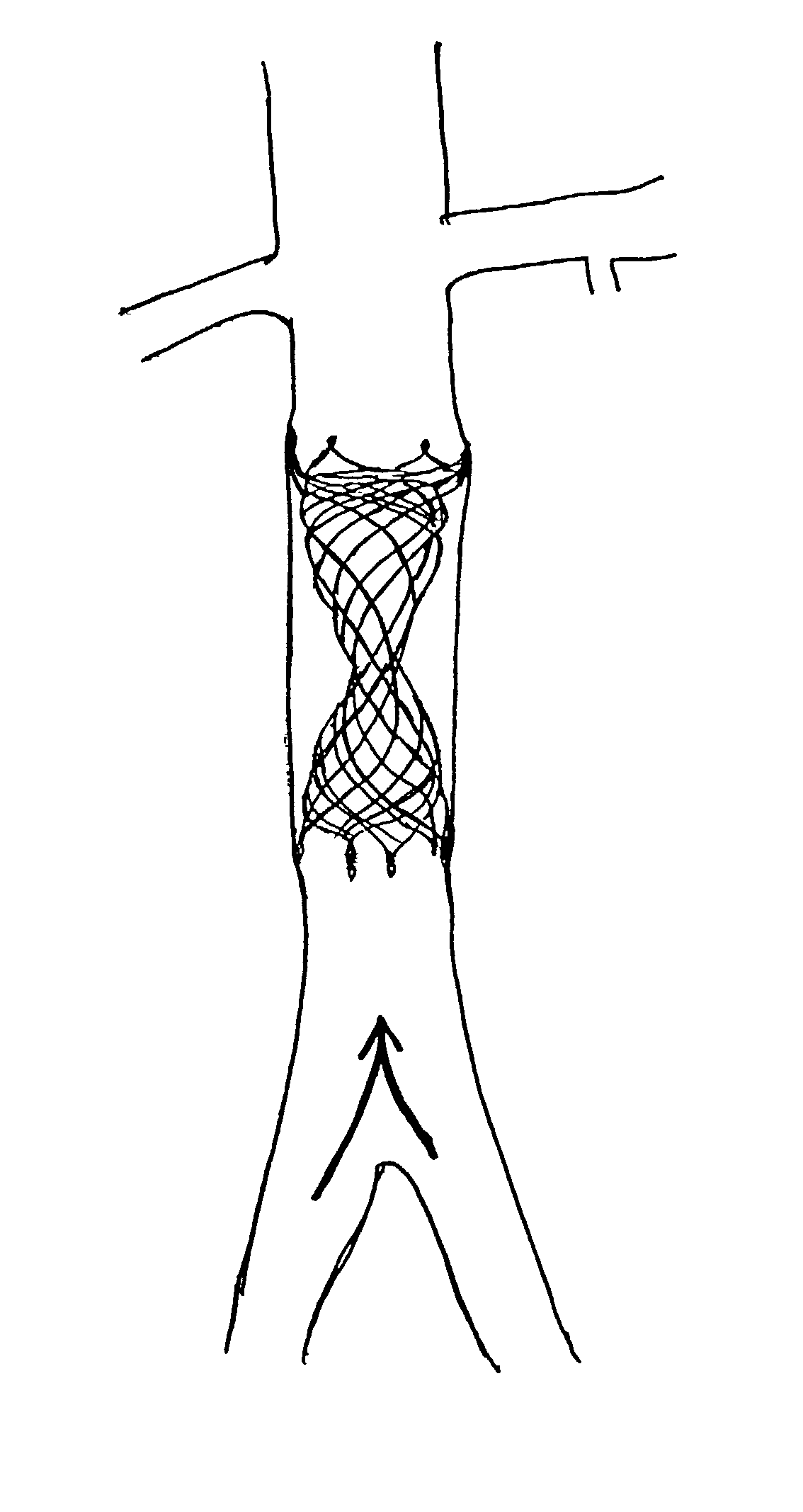
A device and method for treating pathological narrowing of fluid-carrying conduits of the human body (such as blood vessels) in an area of a bifurcation is disclosed. In particular, a stent system carries a self expandable noncylindrical stent, which is particularly suited for treating a widened portion of a blood vessel immediately proximal to a bifurcation. A stent delivery system is also disclosed, for delivering the stent such that a larger expanded diameter end of the stent faces the bifurcation, and a smaller expanded diameter end of the stent faces proximally in the main vessel.
Owner:BIOSENSORS INT GROUP
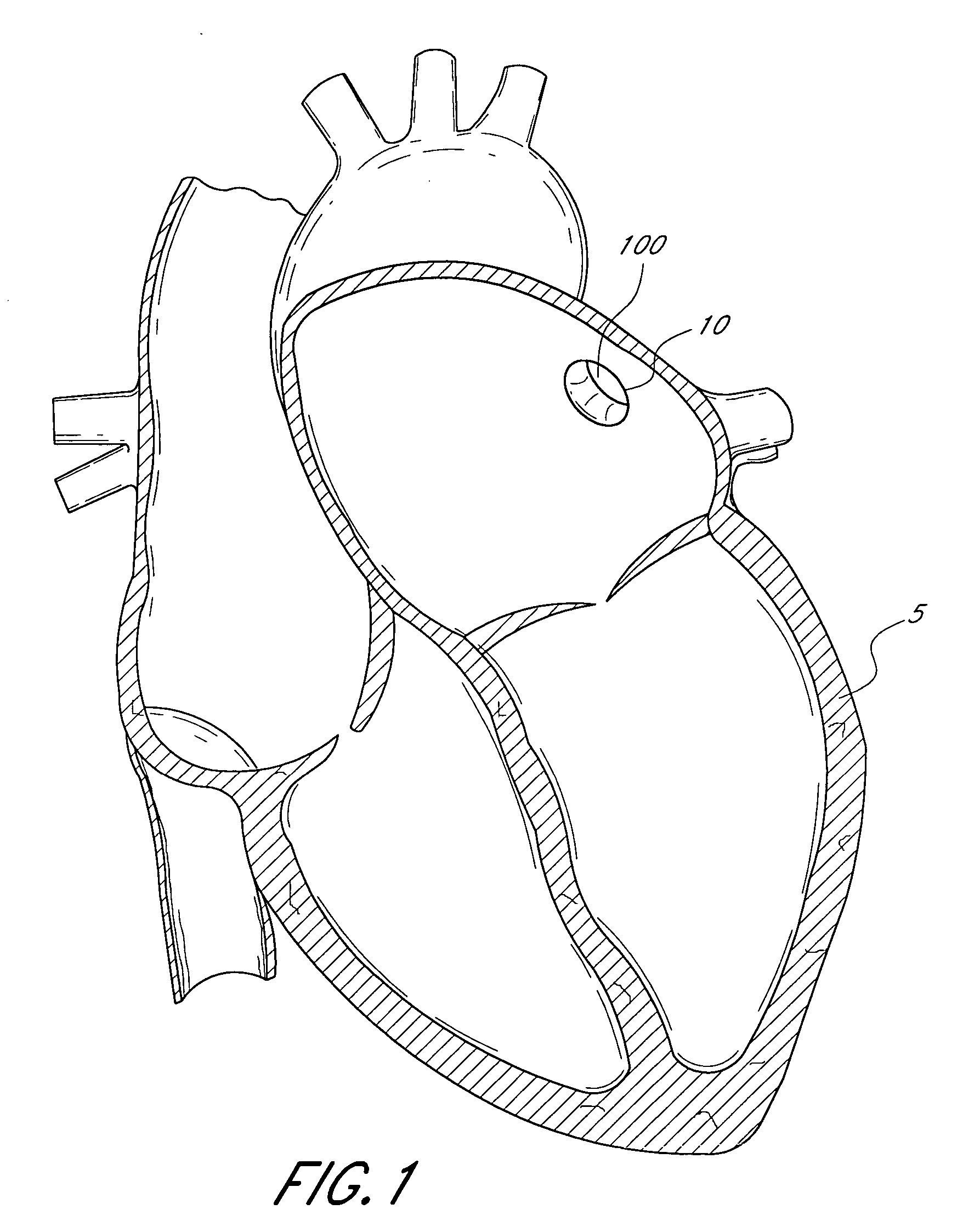
Self-expandable medical instrument for treating defects in a patient's heart
Owner:JENAVALVE TECH INC
Sigmoid valve and method for its percutaneous implantation
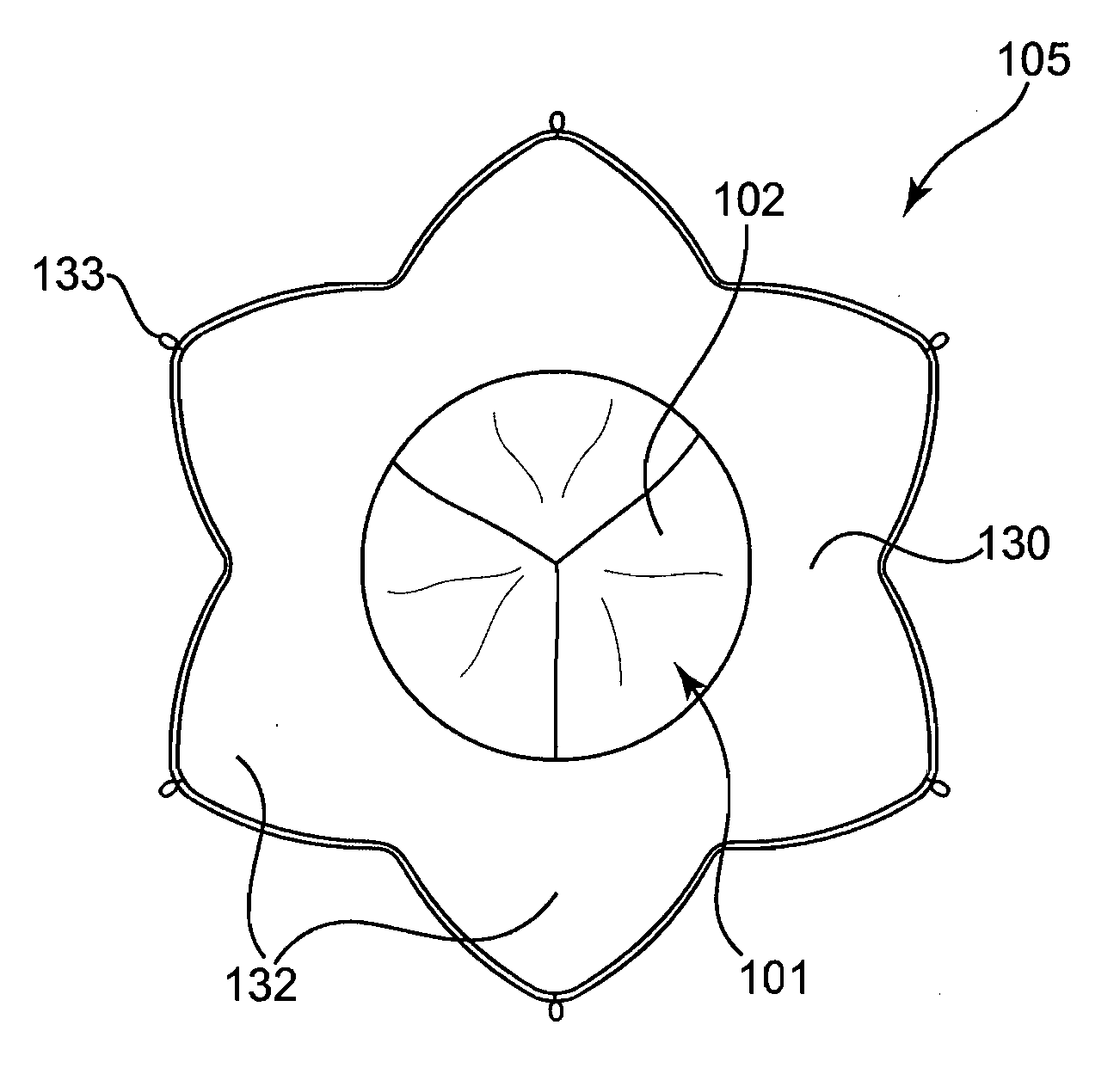
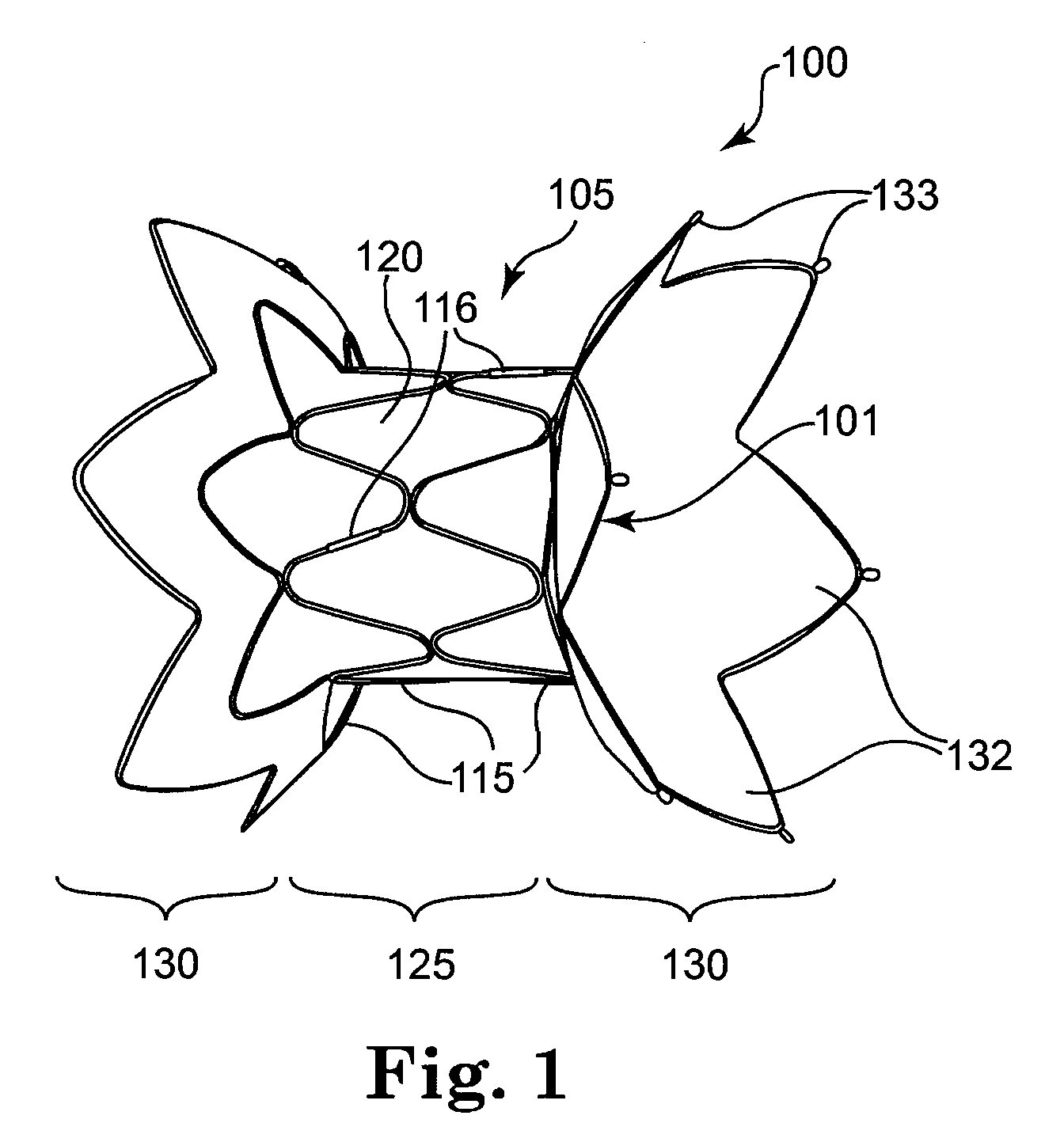
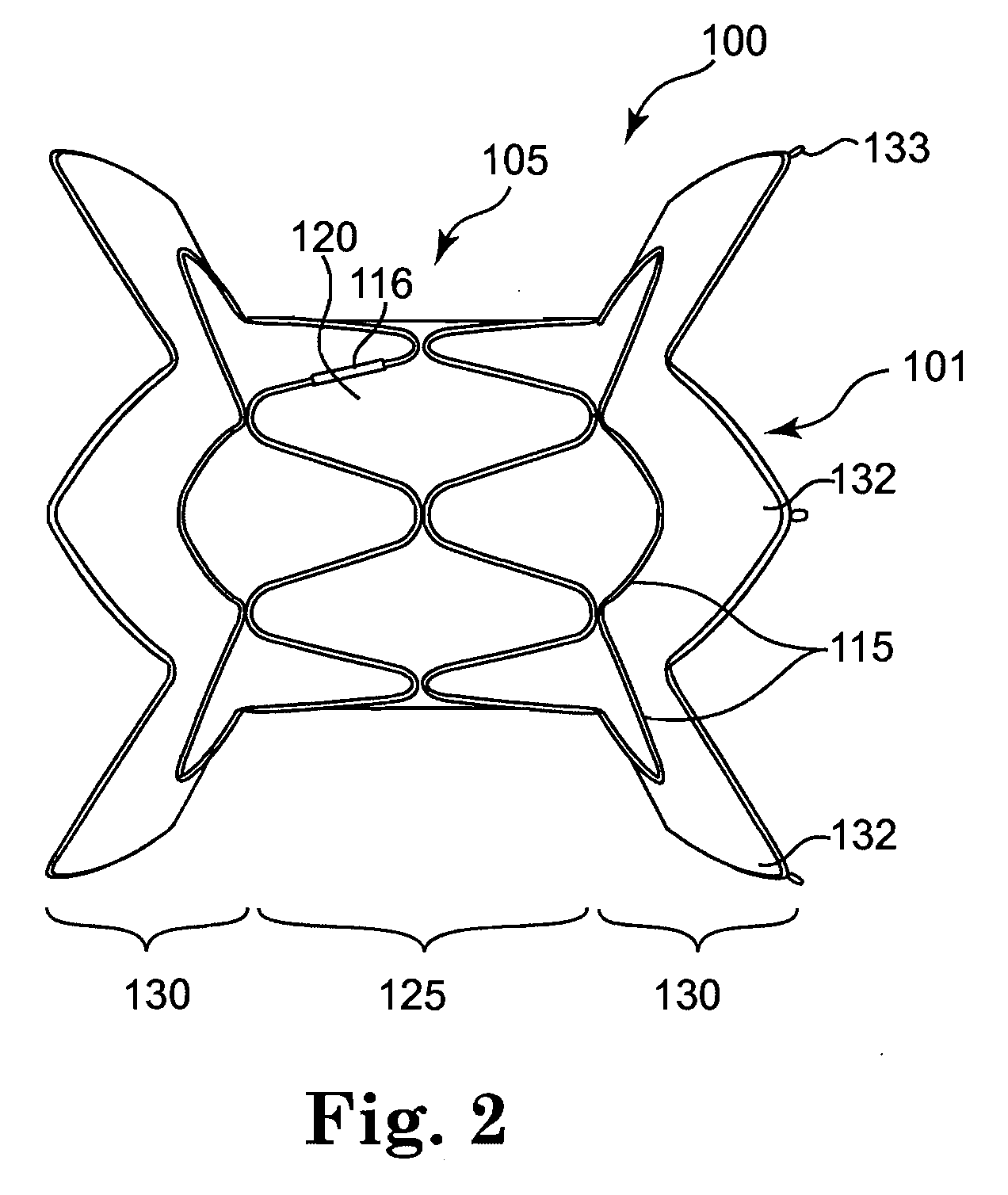
A multi-leaflet valve adapted to serve as a prosthesis for diseased native valve of a mammal is incorporated in self-expandable or inflatable endovascular stents or stents to form a combination which is introduced on a catheter with a guide wire into the circulatory system of the mammal to replace the diseased native valve. Once the combination is at the desired location the stent is caused to expand and affix itself to the patient's vessel wall. The prosthetic valve has the shape of a truncated cone that has an inflow and an outflow orifice with leaflets forming the outflow orifice and forming a plurality of commissures. A first flexible circular support is affixed in a substantially circular fashion around the truncated cone in proximity of the inflow orifice, and a second flexible circular support is affixed at the location of the commissures to form a circle around the truncated cone in proximity of the outflow orifice. The circular supports maintain the shape of the valve during the surgical implantation procedure and thereafter.
Owner:THE INT HEART INST OF MONTANA FOUND
Methods for creating woven devices
Self-expandable, woven intravascular devices for use as stents (both straight and tapered), filters (both temporary and permanent) and occluders for insertion and implantation into a variety of anatomical structures. The devices may be formed from shape memory metals such as nitinol. The devices may also be formed from biodegradable materials. Delivery systems for the devices include two hollow tubes that operate coaxially. A device is secured to the tubes prior to the implantation and delivery of the device by securing one end of the device to the outside of the inner tube and by securing the other end of the device to the outside of the outer tube. The stents may be partially or completely covered by graft materials, but may also be bare. The devices may be formed from a single wire. The devices may be formed by either hand or machine weaving. The devices may be created by bending shape memory wires around tabs projecting from a template, and weaving the ends of the wires to create the body of the device such that the wires cross each other to form a plurality of angles, at least one of the angles being obtuse. The value of the obtuse angle may be increased by axially compressing the body.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods and apparatuses for deploying minimally-invasive heart valves
A system for delivering and deploying a self-expandable heart valve includes a deployment mechanism that engages the valve and regulates the rate of expansion of both the proximal and distal ends thereof. The deployment mechanism may include a plurality of distal fingers and a plurality of proximal fingers that engage the end portions of the heart valve. Controlled radial movement of the fingers regulates the expansion of the heart valve such that the proximal and distal ends radially expand at the same rate. A stabilization balloon may be used to axially and radially locate the deployment mechanism relative to the site of implantation. Methods of operation of the delivery and deployment mechanism include regulating the rate of self-expansion of the valve and forcing the valve outward into its fully expanded configuration utilizing the same or different means.
Owner:EDWARDS LIFESCIENCES CORP
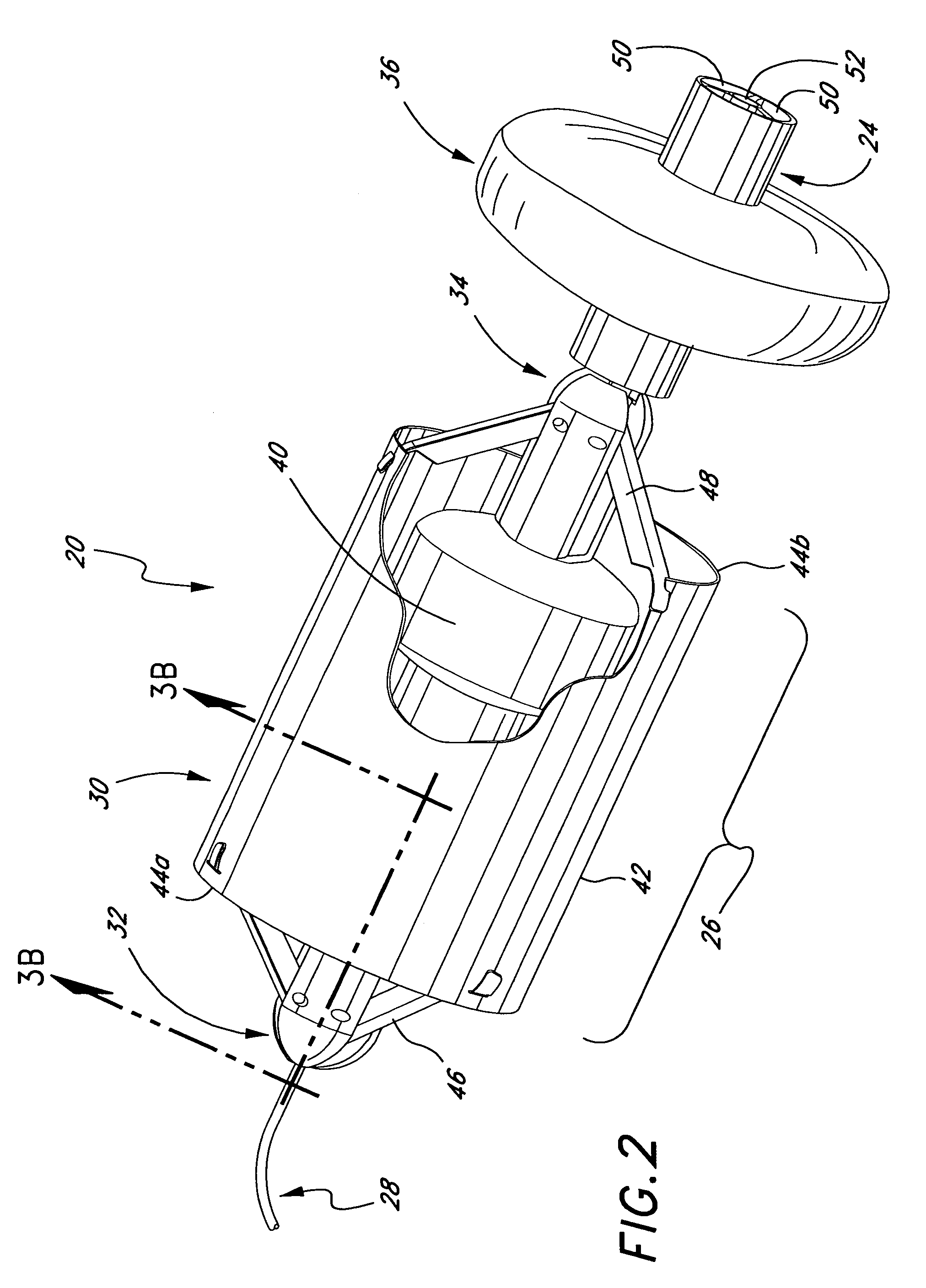
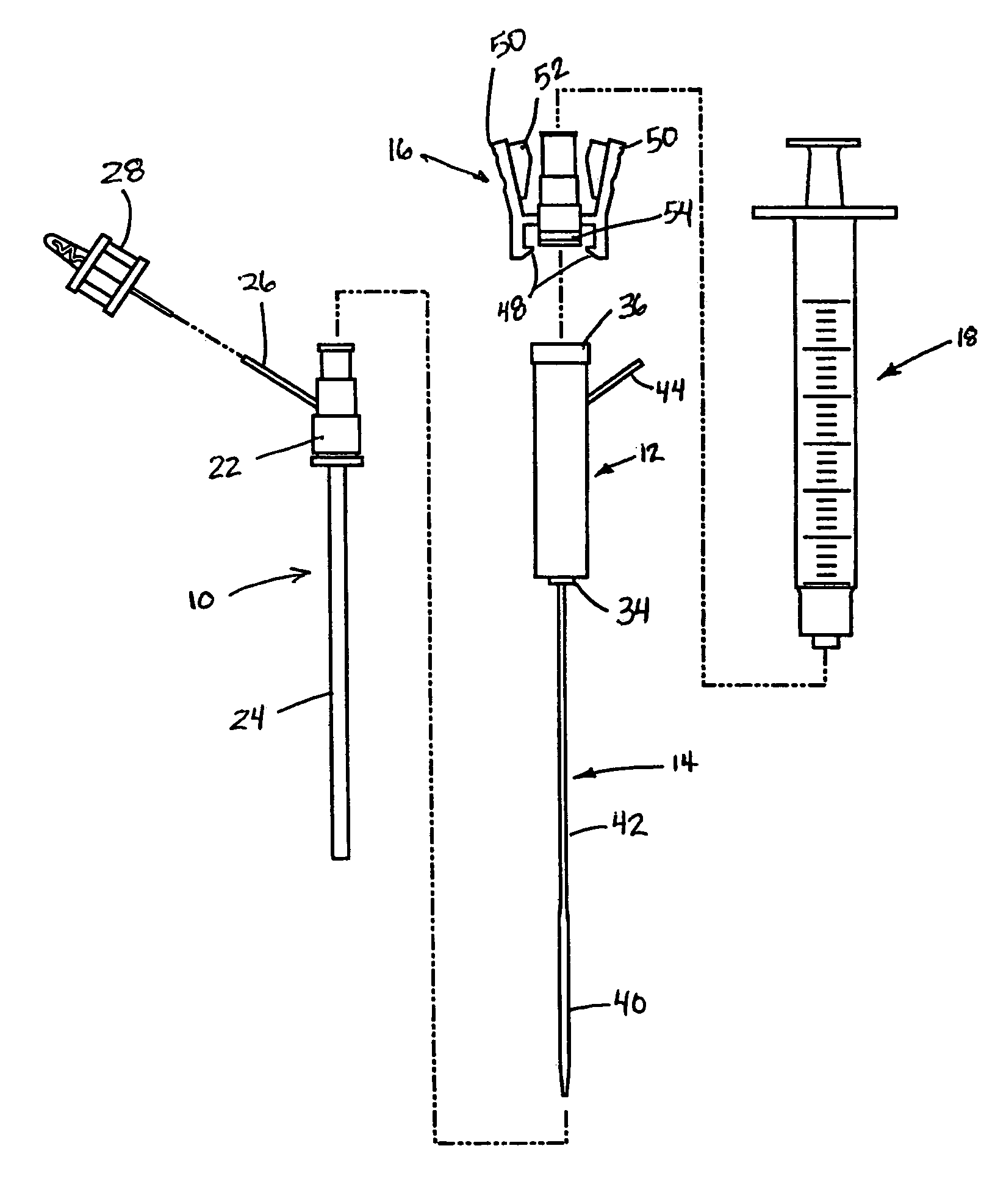
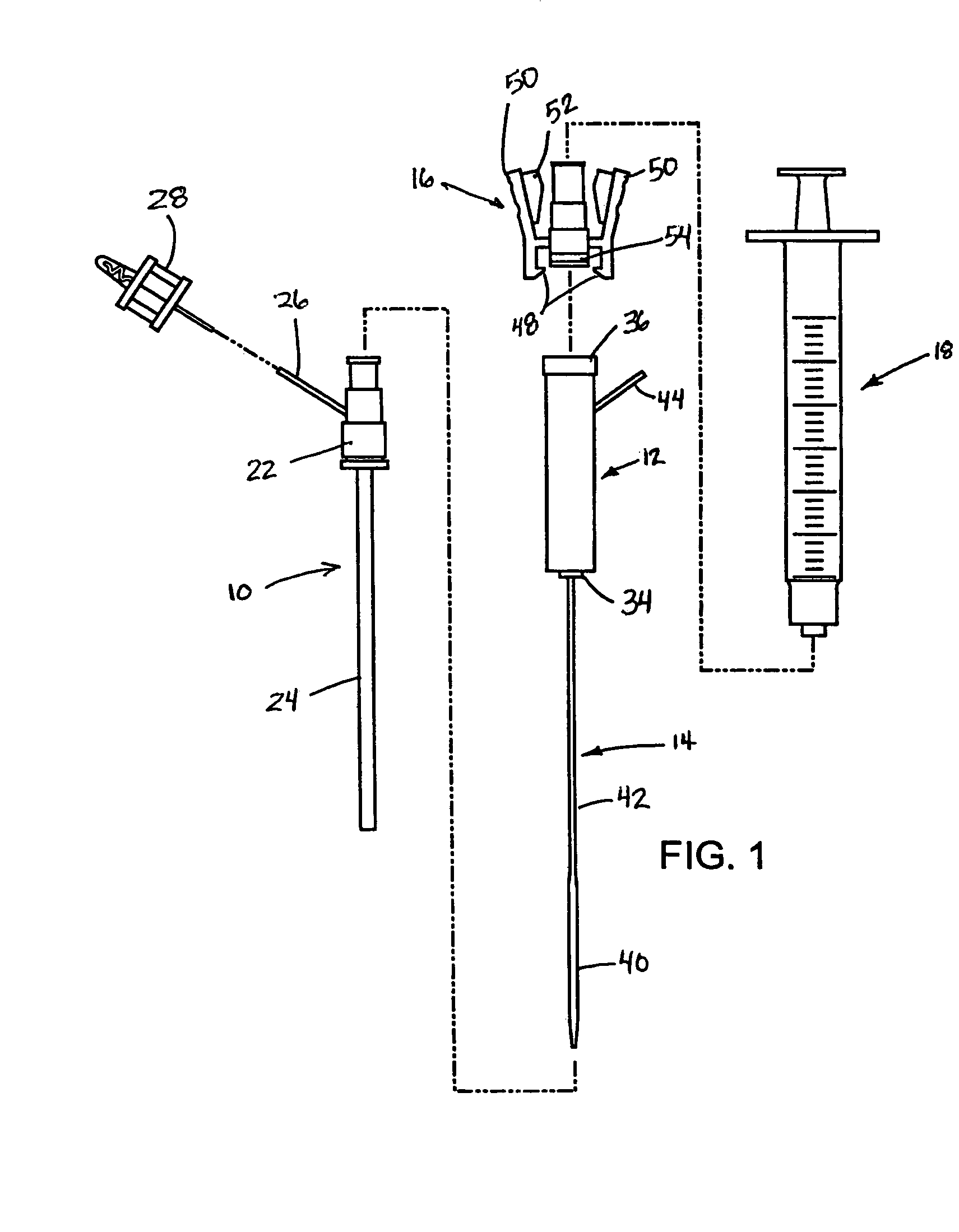
Deployment system for intraluminal devices
ActiveUS20060015171A1Short working lengthRisk minimizationStentsDiagnosticsProsthesisBiomedical engineering
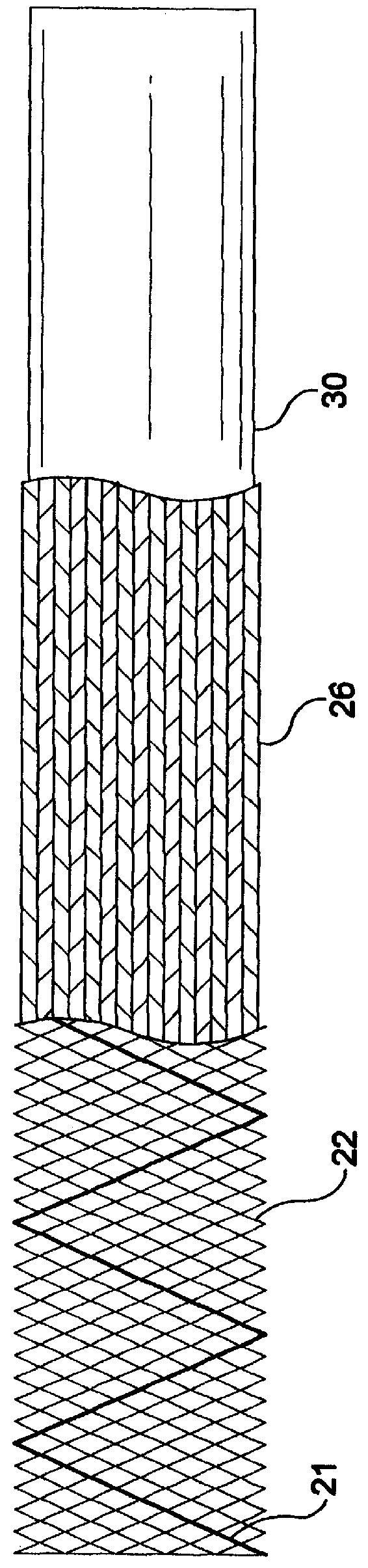
A constraining sheath for use around an endoprosthesis (e.g., a stent device, with or without a graft covering), which may be a balloon expandable endoprosthesis but more preferably is a self-expanding prosthesis. The endoprosthesis is coaxially enclosed within and substantially covered by the constraining sheath, which is an outer, removable tubular sheath, preferably made of ePTFE. The sheath is preferably corrugated circumferentially along at least a portion of the length of the endoprosthesis. The constraining sheath and endoprosthesis are preferably mounted together as an assembly at the distal end of a delivery means such as a catheter shaft, for delivery of the endoprosthesis to a desired location within a body conduit such as an artery. The constraining sheath is removed by the application of tension to a tensile member such as a tether to cause sequential pulling out of the corrugations followed by release and deployment of the endoprosthesis. The use of a corrugated constraining sheath in comparison to a non-corrugated sheath results in a more smoothly applied tensile force to effect the endoprosthesis release as well as requiring less maximum force.
Owner:WL GORE & ASSOC INC
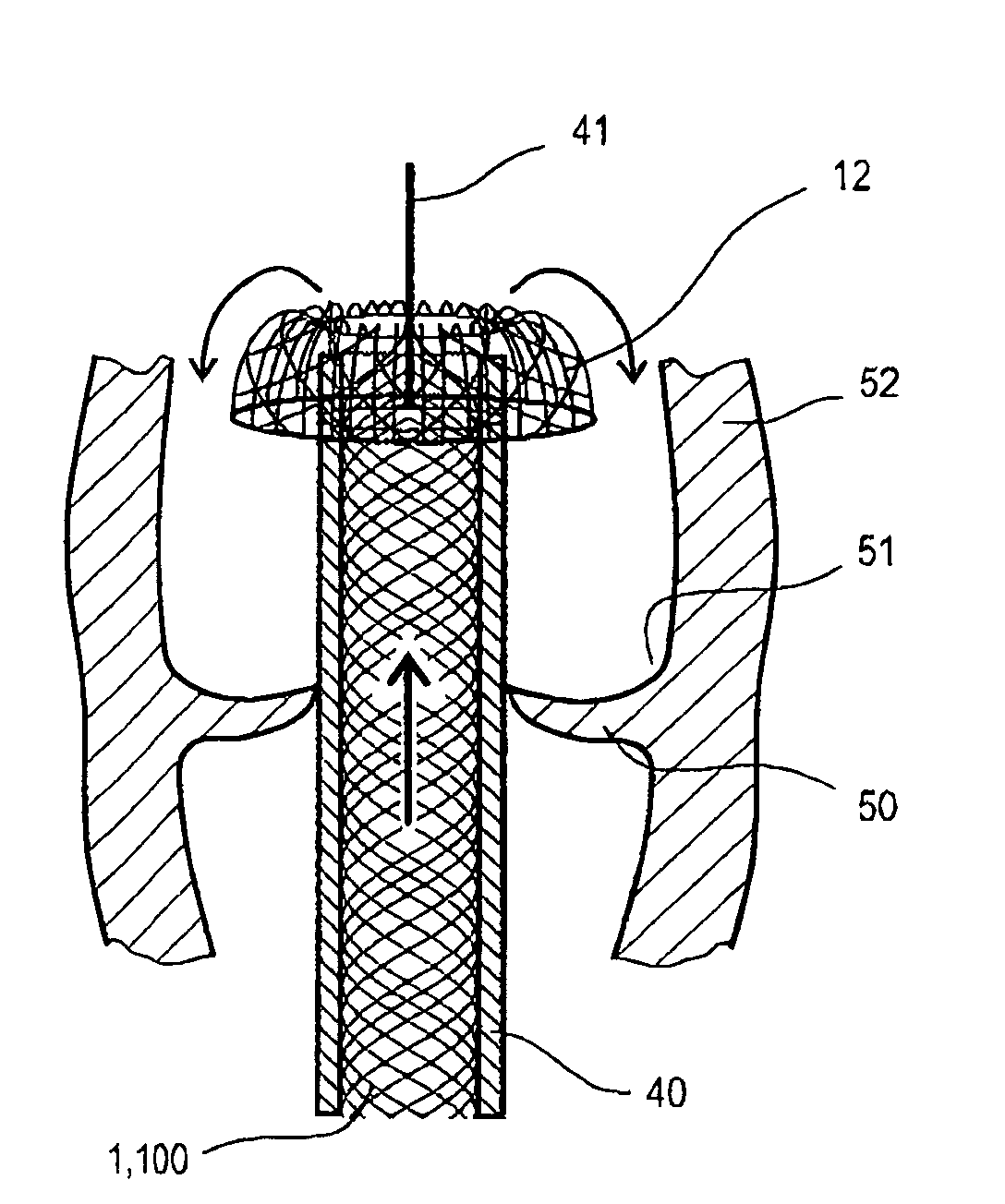
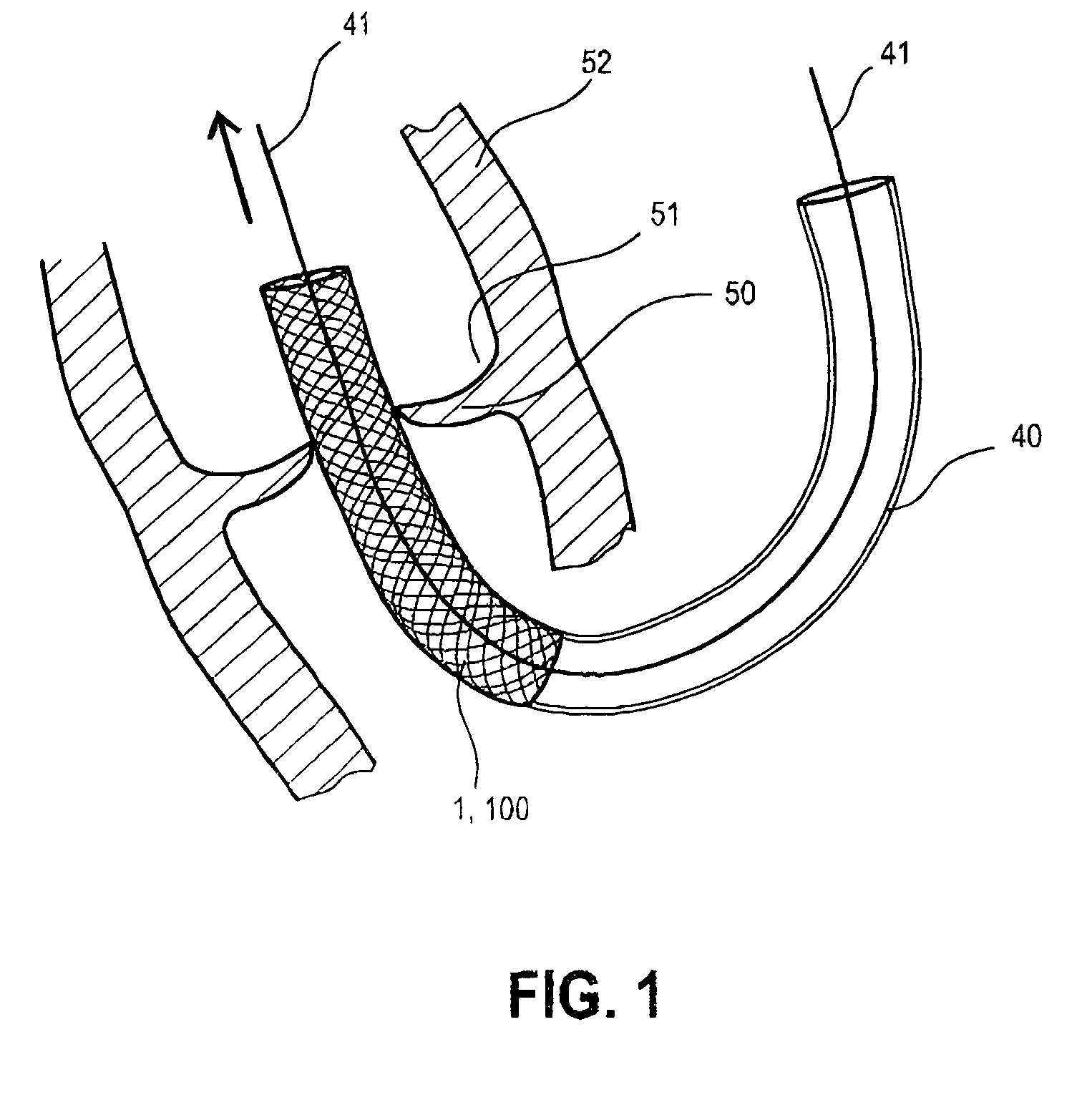
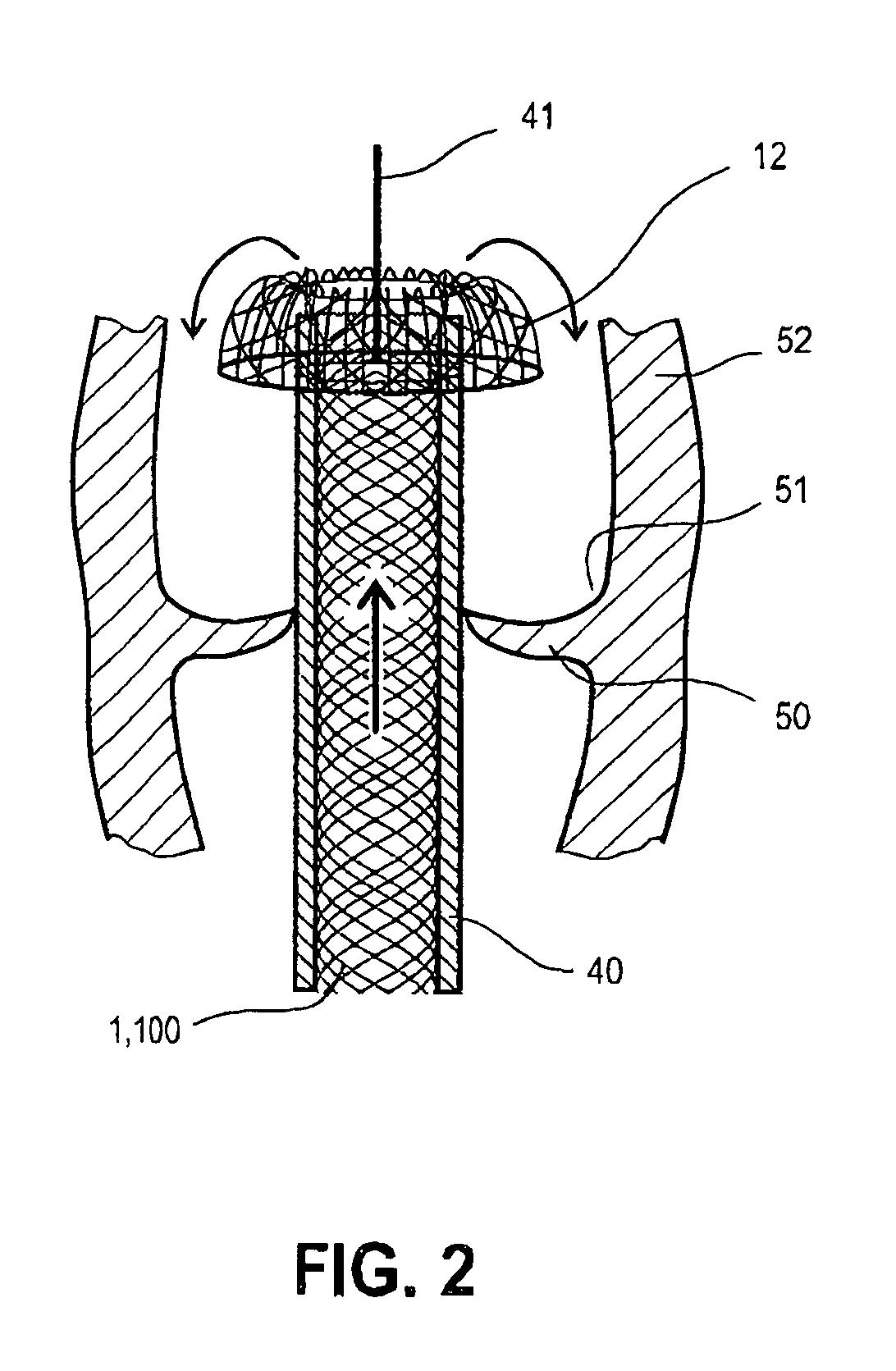
Method and apparatus for delivering an implant without bias to a left atrial appendage
InactiveUS20070135826A1Substantially eliminating,Eliminating implantation biasSuture equipmentsOcculdersImplanted deviceCatheter
A system and method for delivering an implant includes an implant, an actuation shaft, and a concentrically attachable disconnect mount. A distal guide tube is sometimes also provided. A proximal guide tube is also sometimes provided. The implantable device has a proximal, a distal end, and a plurality of supports. The implantable device is moveable between a collapsed and an expanded configuration. The distal guide tube, when provided, is at the distal end of the supports and extends toward the proximal end of the implantable device. The actuation shaft extends through the proximal end of the implantable device and is removeably engageable with the distal guide tube, or the distal end of the device when the distal guide tube is not provided. The disconnect mount is releasably engageable with the proximal end of the implantable device. The disconnect mount is concentrically attachable to the proximal end of the implantable device as well. The implantable device is self-expandable, and is collapsed by engaging the actuation shaft with the distal guide tube while applying a relatively proximal force to the proximal end of the implant with the disconnect mount.
Owner:BOSTON SCI SCIMED INC
Stent-graft-membrane and method of making the same
A braided self-expandable stent-graft-membrane made of elongated members forming a generally tubular body. A membrane layer and graft layer are disposed on a endoprosthesis such as a stent. The membrane layer is substantially impermeable to fluids. The outermost layer is biocompatible with the body tissue. The innermost layer is biocompatible with the fluid in the passage. An embodiment includes a graft layer disposed on the inside of a stent and a membrane layer disposed on the outside of the stent. The innermost layer is biocompatible with the fluid in the passage. The stent-graft-membrane is used at a treatment site in a body vessel or organ where it is desirous to exclude a first fluid located outside the endoprosthesis from reaching a second fluid located in the lumen. The membrane may be made of silicone or polycarbonate urethane. The graft may be braided, woven, spun or spray-cast PET, PCU, or PU fibers. The layers may include ePTFE or PTFE.
Owner:LIFEPORT SCI
Percutaneous valve prosthesis and system and method for implanting same
InactiveUS20090306768A1Minimizes gradientIncrease the areaHeart valvesBlood vesselsInsertion stentBalloon dilatation
A heart valve prosthesis includes a cylindrical valve cage stent constructed to be implanted percutaneously in the planar axis of a native valve annulus, an elastic and compressible, multi-leaflet valve insertable percutaneously into the body, and an attachment mechanism for attaching the valve to the superior rim of the valve cage stent. The valve can be of a bi-leaflet or a tri-leaflet type and includes a valve frame made from a memory metal and a tissue cover attached to the valve frame. The valve cage stent is self-expanding or balloon expandable, made respectively from memory metal or stainless steel but otherwise structurally the same.
Owner:EDWARDS LIFESCI CARDIAQ
Infundibular Reducer Devices
ActiveUS20100049306A1Easy to explainEliminate failure problemsStentsHeart valvesProsthetic valveInsertion stent
Described is a prosthetic valve assembly comprising: a radially self-expandable stent configured to expand to bear against a wall of a native body lumen; and an implantable prosthetic valve, having a diameter, the valve being mounted inside the stent; wherein the diameter of the stent is greater than the diameter of the prosthetic valve.
Owner:MEDTRONIC VASCULAR INC
Biocompatible crosslinked coating and crosslinkable coating polymer composition for forming such a coating
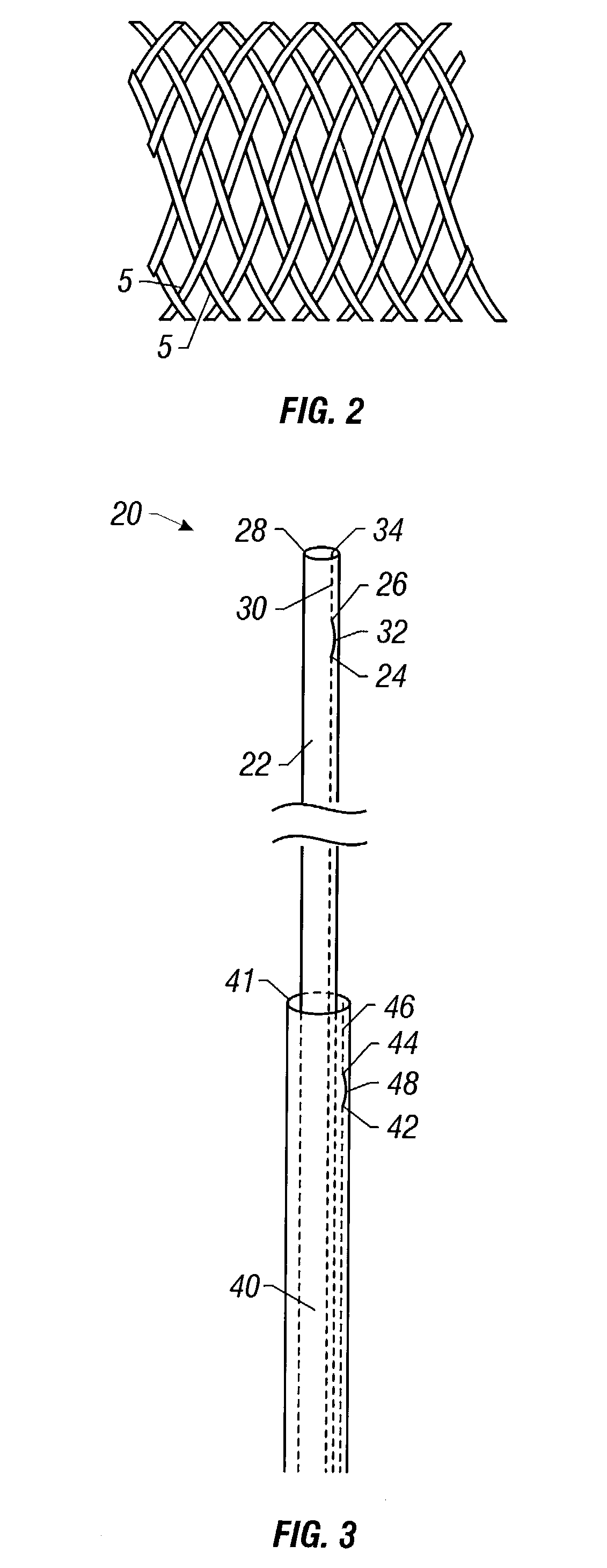
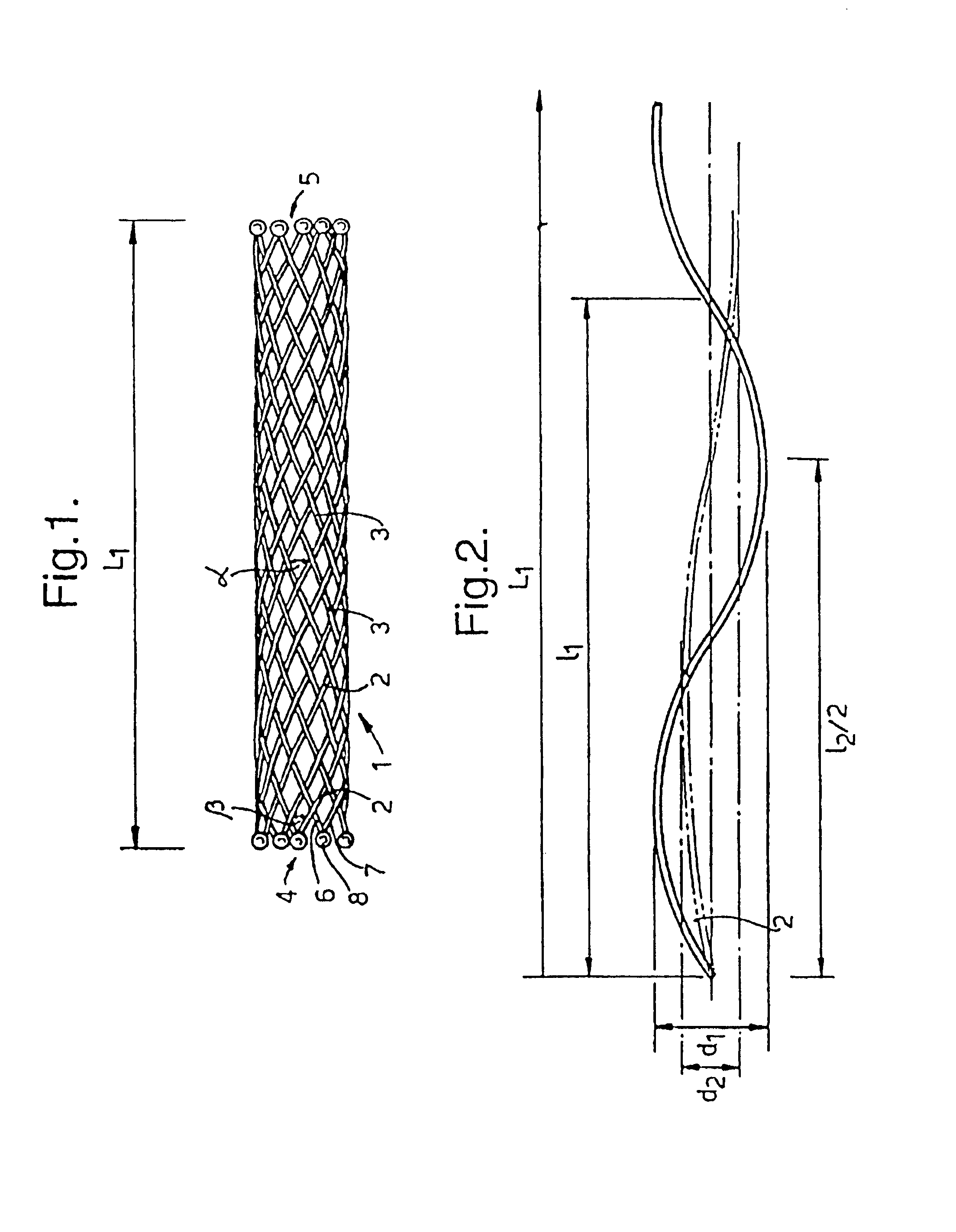
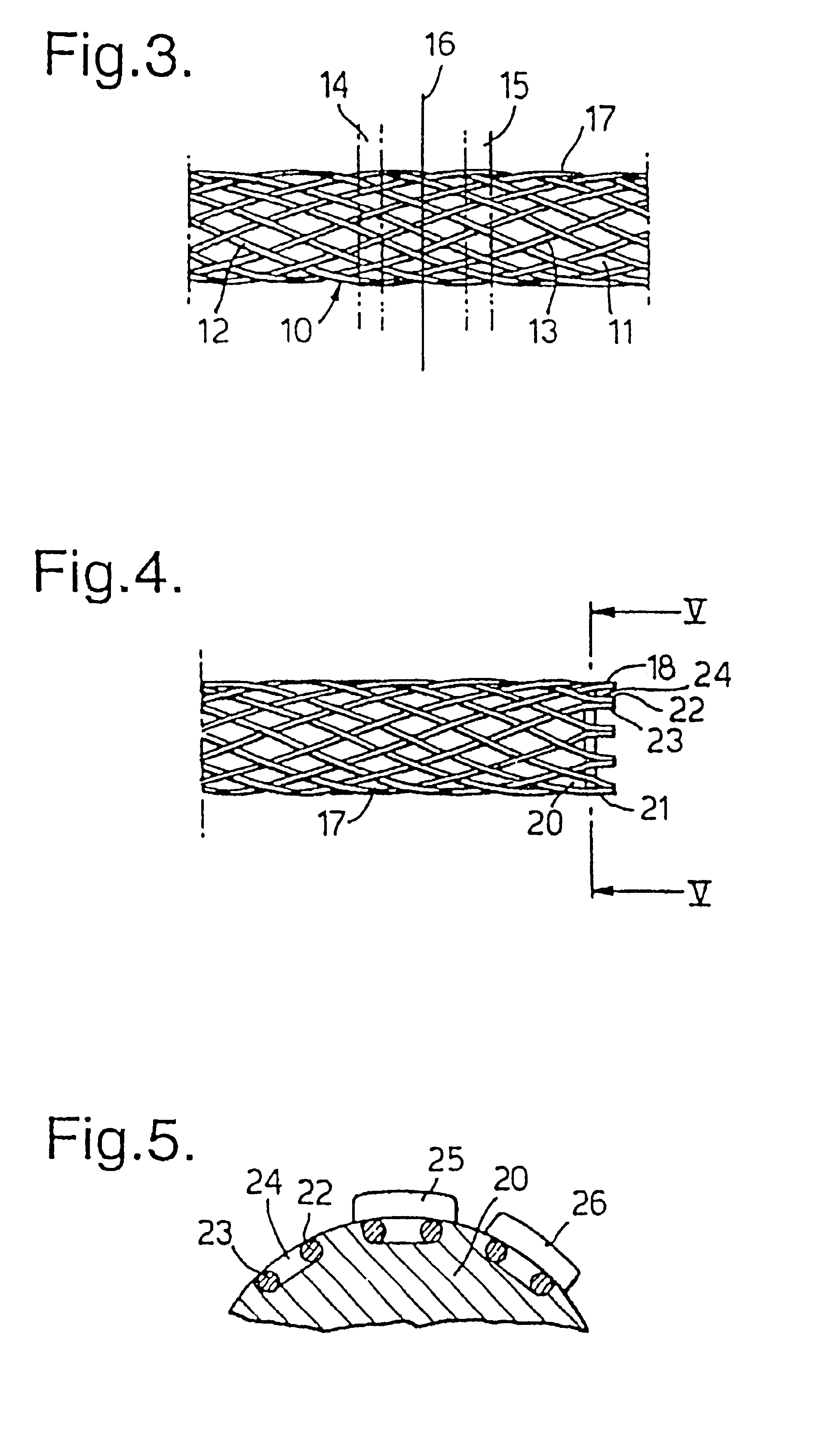
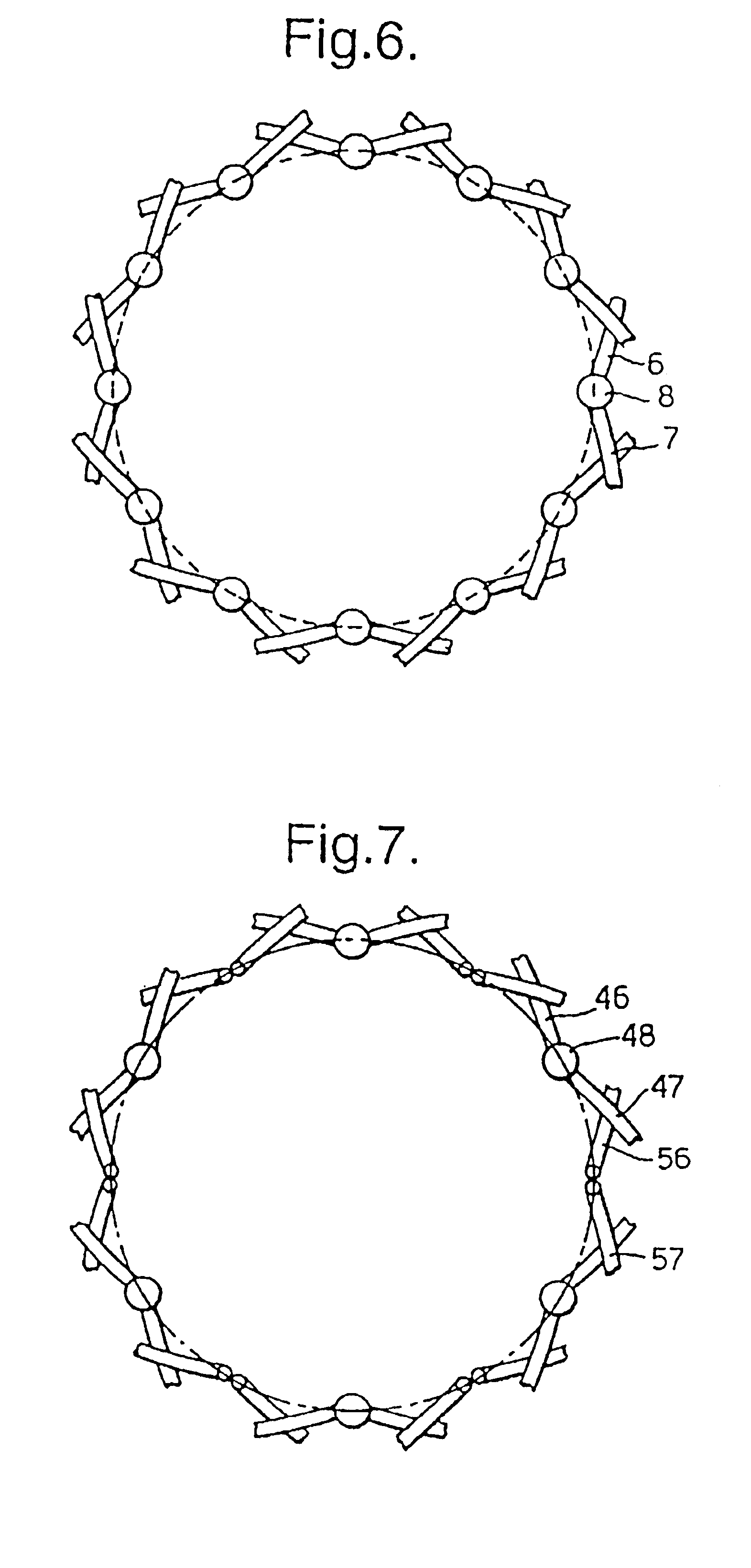
A braided stent (1) for transluminal implantation in body lumens is self-expanding and has a radial expanded configuration in which the angle α between filaments is acute. Some or all of filaments (6,7) are welded together in pairs at each end (4,5) of the stent to provide beads (8), thereby strengthening the stent and assisting its deployment from a delivery device. The stent is preferably completely coated using a biocompatible polymeric coating, said polymer preferably having pendant phosphoryl choline groups. A method of making the stent by braiding and welding is described as well as a delivery device for deploying the device.The present invention provides a biocompatible crosslinked coating and a crosslinkable coating polymer composition for forming such a coating. The biocompatible crosslinked coating may be formed by curing a polymer of 23 mole % (methacryloyloxy ethyl)-2-(trimethylammonium ethyl) phosphate inner salt, 47 mole % lauryl methacrylate, 5 mole % γtrimethoxysilyl propyl methacrylate and 25 mole % of hydroxy propyl methacrylate. The crosslinkable coating polymer may include 23 mole % (methacryloyloxy ethyl)-2-(trimethylammonium ethyl) phosphate inner salt, 47 mole % lauryl methacrylate, 5 mole % γtrimethoxysilyl propyl methacrylate and 25 mole % of hydroxy propyl methacrylate.<?insert-end id="INS-S-00001" ?>
Owner:BIOCOMPATIBLES UK LTD
Cardiac valve annulus restraining device
A catheter based system for treating mitral valve regurgitation includes a reshaping device having a body and a plurality of movable anchoring barbs attached to the body of the device. The reshaping device can be made from a biocompatible material having suitable shape memory properties. The devices of the current invention can be self expandable, balloon expandable, or a combination self expandable and balloon expandable. One embodiment of the invention includes a method for attaching a reshaping device to the annulus of a mitral valve, moving the body of the device from a fully expanded configuration to a resting configuration, and thereby reshaping the mitral valve annulus.
Owner:MEDTRONIC VASCULAR INC
Stent system for preventing restenosis
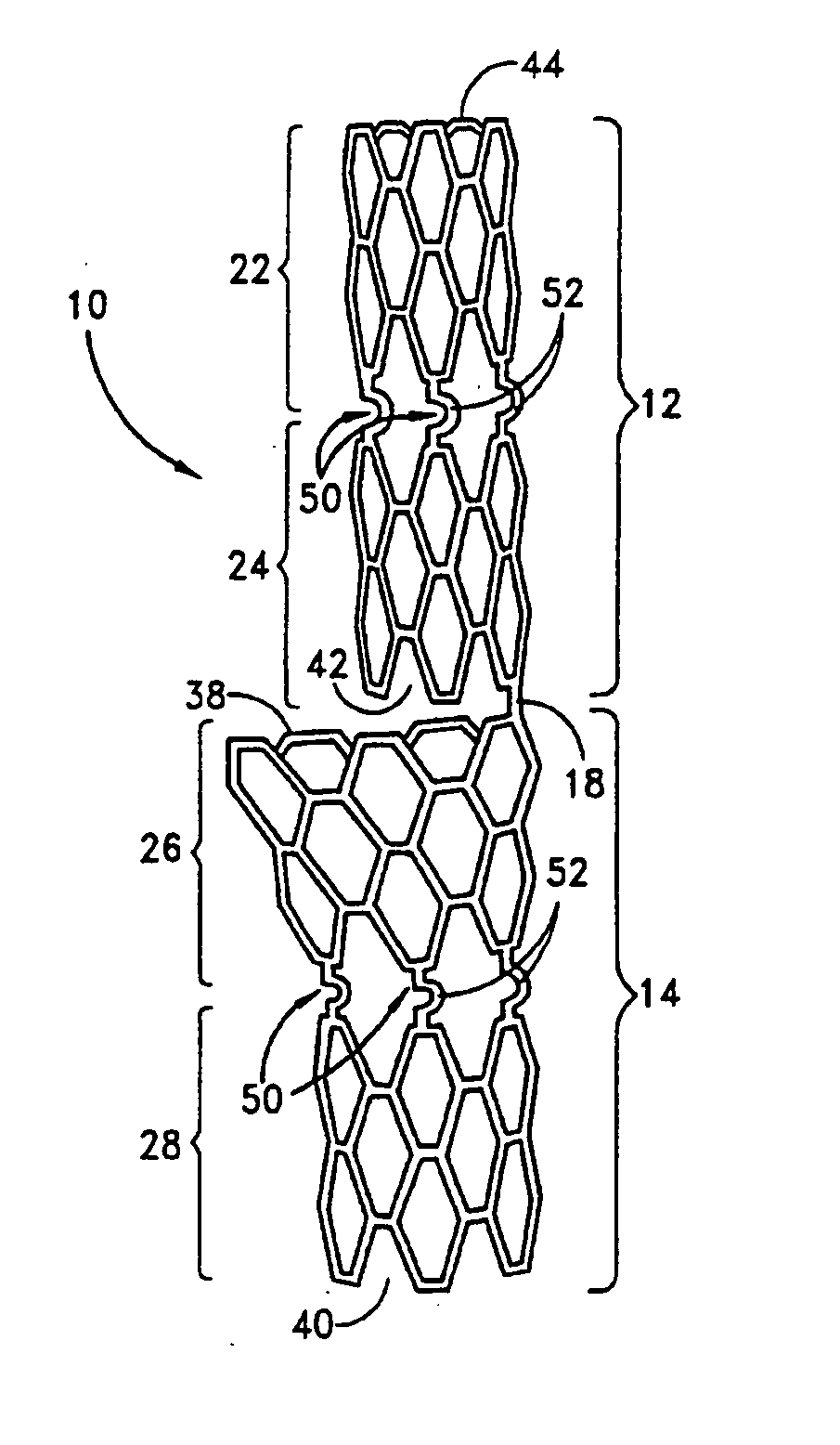
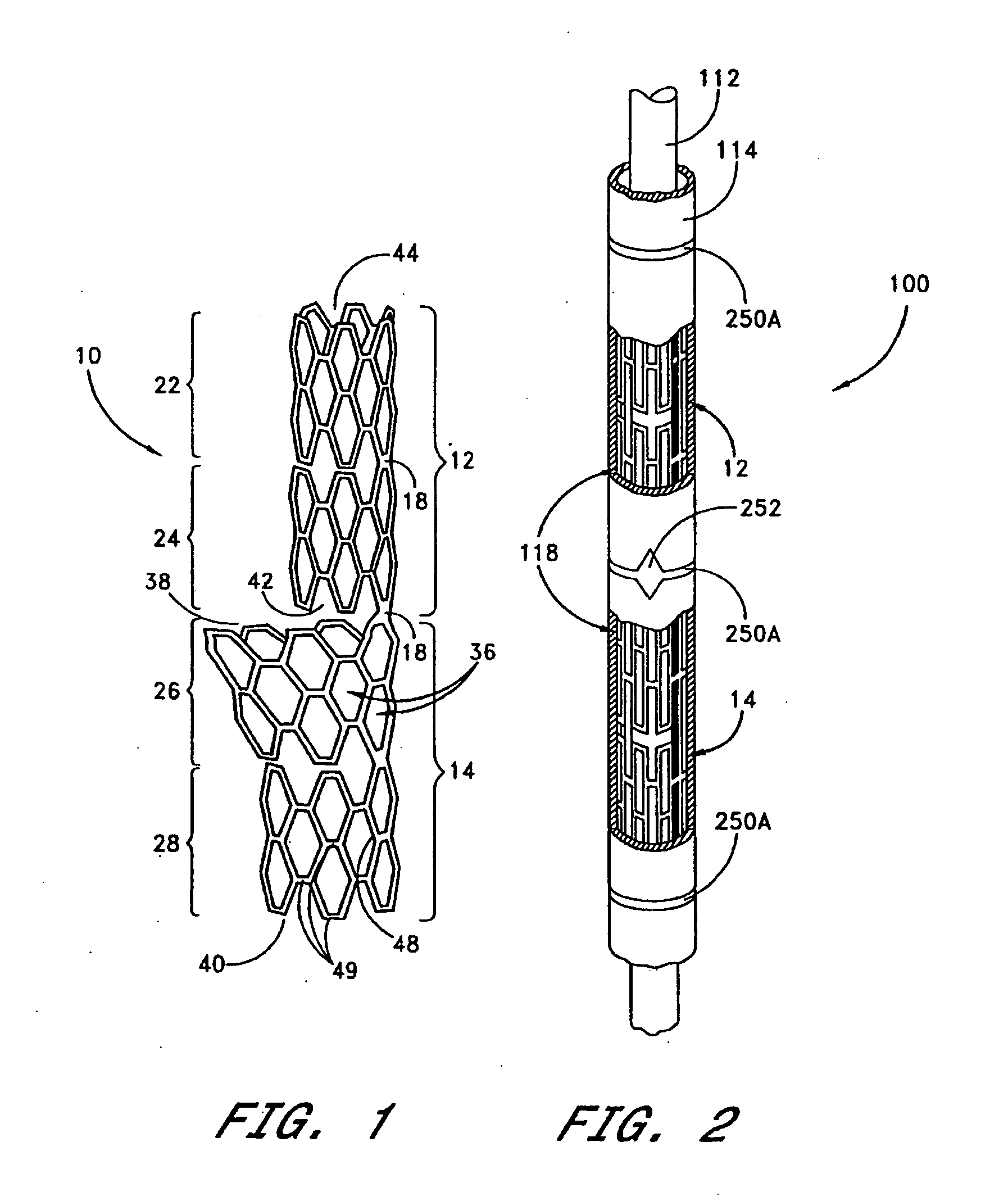
A system for treating a body lumen is disclosed. The system comprises an outer stent and an inner stent disposed within the lumen of the outer stent. At least one end of the inner stent extends outside of the lumen of the outer stent, so that the end of the inner stent contacts and conforms to the body lumen wall that is adjacent the end of the outer stent. A coating can be disposed on a surface, preferably the outer surface, of the inner stent. The coating contains a therapeutic substance that may be released into the body lumen wall to help in preventing restenosis. Also disclosed is a stent having a balloon-expandable portion connected to a self-expanding portion. Methods for deploying the system and the stent are also disclosed.
Owner:BOSTON SCI SCIMED INC
Infundibular reducer devices
Described is a prosthetic valve assembly comprising: a radially self-expandable stent configured to expand to bear against a wall of a native body lumen; and an implantable prosthetic valve, having a diameter, the valve being mounted inside the stent; wherein the diameter of the stent is greater than the diameter of the prosthetic valve.
Owner:MEDTRONIC VASCULAR INC
Satiation devices and methods
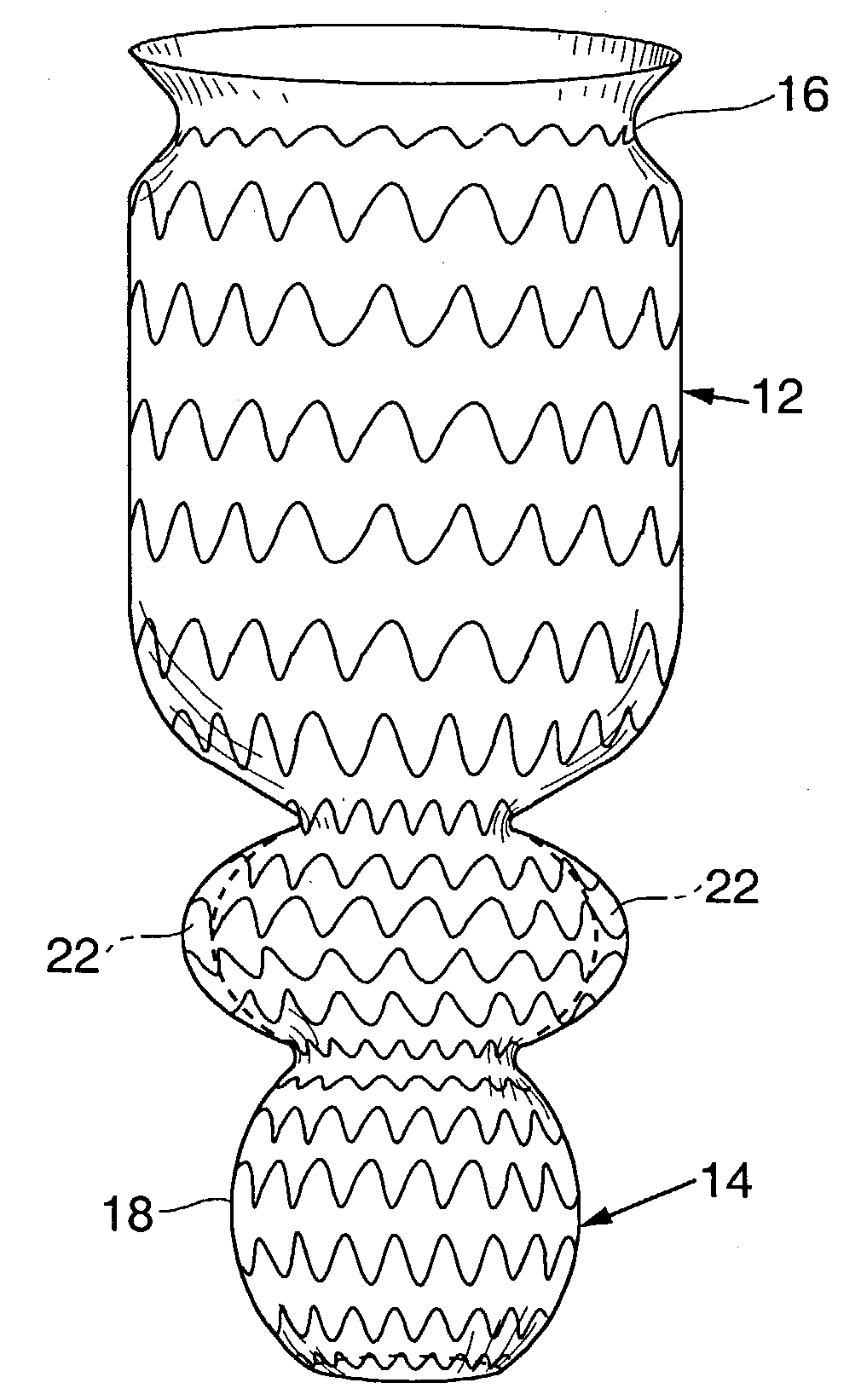
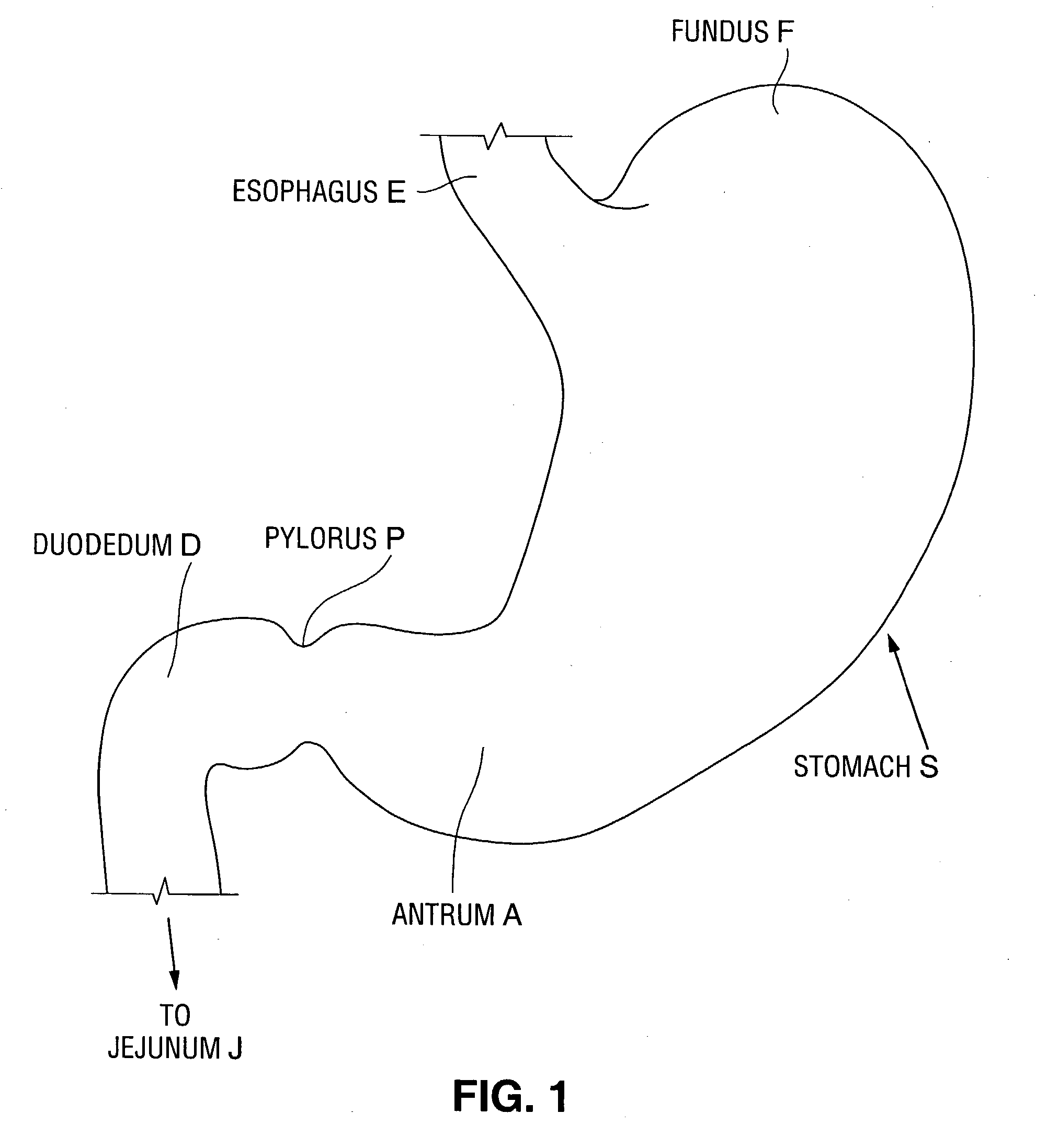
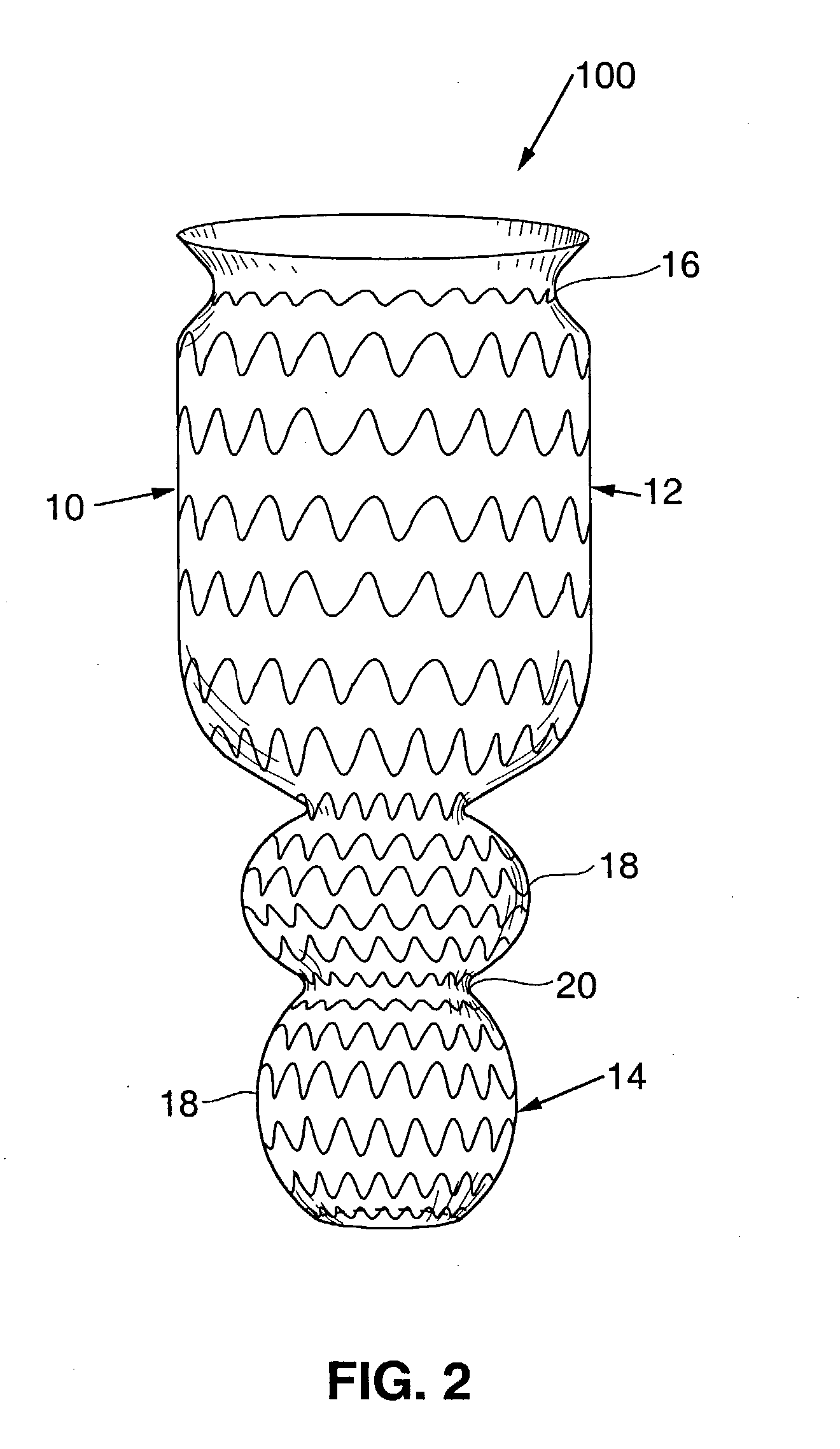
A device for inducing weight loss in a patient includes a tubular prosthesis self-expandable from a collapsed position in which the prosthesis has a first diameter to an expanded position in which the prosthesis has a second, larger, diameter. In a method for inducing weight loss, the prosthesis is placed in the collapsed position and inserted into a stomach of a patient. The prosthesis is allowed to self-expand from the collapsed position to the expanded position and into contact with the walls of the stomach, where it induces feelings of satiety and / or inhibits modulation of satiety-controlling factors such as Ghrelin.
Owner:BOSTON SCI SCIMED INC
Self-expandable aneurysm filling device, system and method of placement
The self-expandable aneurysm filling device, system and method provide for placement of the stent into an aneurysm to at least partially fill and stabilize the aneurysm. The self-expandable aneurysm filling device has a compressed undeployed configuration and an expanded three-dimensional deployed configuration, and a severable deployment junction releasably connects the self-expandable aneurysm filling device to a pusher wire. The severable deployment junction can be mechanically, electrolytically, or thermally severed to separate the self-expandable aneurysm filling device from the pusher wire.
Owner:PENUMBRA
Flexible stent and method of making the same
InactiveUS7288111B1Increasing the thicknessFlexible connectionStentsBlood vesselsPolymerSelf expandable
Owner:TC1 LLC
Sigmoid valve and method for its percutaneous implantation
Owner:THE INT HEART INST OF MONTANA FOUND
Atrial Thrombogenic Sealing Pockets for Prosthetic Mitral Valves
This invention relates to a self-expanding pre-configured compressible transcatheter prosthetic cardiovascular valve that comprises atrial thrombogenic sealing pocket cover mounted on a self-expanding inner wire frame having a leaflet structure comprised of articulating leaflets that define a valve function, said inner wire frame is disposed within a self-expanding annular tissue-covered outer wire frame, said outer wire frame having an articulating collar, forming a multi-component prosthetic valve assembly for anchoring within the mitral valve or triscuspid valve of the heart, and methods for deploying such a valve for treatment of a patient in need thereof.
Owner:TENDYNE HLDG
Pledget-handling system and method for delivering hemostasis promoting material to a blood vessel puncture site by fluid pressure
A system and method for delivering a pledget of hemostasis promoting material to a blood vessel puncture to facilitate hemostasis, having an introducer sheath having an inner diameter and an outer diameter, a control tip coupled to the introducer sheath, and a self-expandable member disposed around a portion of the control tip to control blood flow at the blood vessel puncture.
Owner:BOSTON SCI SCIMED INC +10
Methods and apparatus for flow restoration
Methods for restoring blood flow in occluded blood vessels using an apparatus having a self expandable distal segment that is pre-formed to assume a superimposed structure in an unconstrained condition but can be made to take on a volume-reduced form making it possible to introduce it with a microcatheter and a push wire arranged at the proximal end, with the distal segment in its superimposed structure assuming the form of a longitudinally open tube and having a mesh structure of interconnected strings or filaments or struts. In a preferred embodiment, the distal segment has a tapering structure at its proximal end where the strings or filaments or struts converge at a connection point.
Owner:TYCO HEALTHCARE GRP LP
Stent-graft-membrane and method of making the same
A braided self-expandable stent-graft-membrane made of elongated members forming a generally tubular body. A membrane layer and graft layer are disposed on a endoprosthesis such as a stent. The membrane layer is substantially impermeable to fluids. The outermost layer is biocompatible with the body tissue. The innermost layer is biocompatible with the fluid in the passage. An embodiment includes a graft layer disposed on the inside of a stent and a membrane layer disposed on the outside of the stent. The innermost layer is biocompatible with the fluid in the passage. The stent-graft-membrane is used at a treatment site in a body vessel or organ where it is desirous to exclude a first fluid located outside the endoprosthesis from reaching a second fluid located in the lumen. The membrane may be made of silicone or polycarbonate urethane. The graft may be braided, woven, spun or spray-cast PET, PCU, or PU fibers. The layers may include ePTFE or PTFE.
Owner:LIFEPORT SCI
Catheter pump
ActiveUS20140012065A1Easy to compressMinimize risk of fractureControl devicesBlood pumpsFar lateralCatheter device
A catheter pump is provided that includes a rotatable impeller and an elongate cannula. The elongate cannula has a mesh that has a plurality of circumferential members disposed about the impeller. The elongate cannula has a plurality of axial connector extending between a proximal side of a distal circumferential member and a distal side of a proximal circumferential member. The circumferential members are radially self-expandable. The cannula is configured to minimize fracture within at least in the distal zone of the mesh as the elongated cannula moves into a sheathing device.
Owner:THORATEC CORPORTION
Noncylindrical stent deployment for treating vascular bifurcations
Owner:BIOSENSORS INT GROUP
Combination self-expandable, balloon-expandable endoluminal device
An endoluminal device, such as a stent or a vena cava filter, comprising at least one superelastic section and at least one plastically deformable section. The superelastic section may comprise, for example, a superelastic grade of nitinol, whereas the plastically deformable section may comprise, for example, gold, platinum, tantalum, titanium, stainless steel, tungsten, a nickel alloy, a cobalt ally, a titanium alloy, or a combination thereof. Each plastically deformable section may merely comprise a constrained portion of the superelastic section comprising a plastically deformable material, such as gold. The device enables deployment by a method comprising introducing the device into a body lumen with the device radially constrained in a first configuration having a first diameter; allowing the device to self-expand into a second configuration having a second diameter less than or equal to a fully-self-expanded diameter; and then optionally “fine-tuning” the device by forcibly expanding the device into a third configuration having a third diameter in a range between the second diameter and less than or equal to a fully-forcibly-expanded diameter. The superelastic and plastically deformable sections may be tubular sections placed end-to-end, such that the plastically deformable section can be conformed to fit a tapered section of a lumen.
Owner:BOSTON SCI SCIMED INC
Stent with controlled expansion
An intraluminal prosthesis composed of a self-expandable stent and a biodegradable constraining element being capable of biodegrading in vivo over a predetermined period of time to permit radial expansion of the stent. The constraining elements are applied to the stent to produce a compressed configuration. Dissolution of the constraining elements in vivo allows for expansion of the stent to an expanded configuration.
Owner:BOSTON SCI SCIMED INC
Clot retrieval devices
A self-expandable mechanical clot retrieval device is designed variously to dislodge, engage and retract blood clot from extremely small and tortuous vasculature. The retrieval device comprises an elongate member and a plurality of ring elements. The ring elements may comprise a plurality of struts and crowns interconnected by a tether formed at a distal end of the elongate member or may be a separate component attached thereto. At least one tether may connect each ring element to the elongate member to restrain the ring element in a collapsed configuration during delivery of the retrieval device through an intravascular microcatheter. The tether connecting the ring element to the elongate member disengages when the retrieval device is positioned at the occluded site and the microcatheter is retracted to allow the self-expandable ring elements to reach an expanded configuration.
Owner:NEURAVI
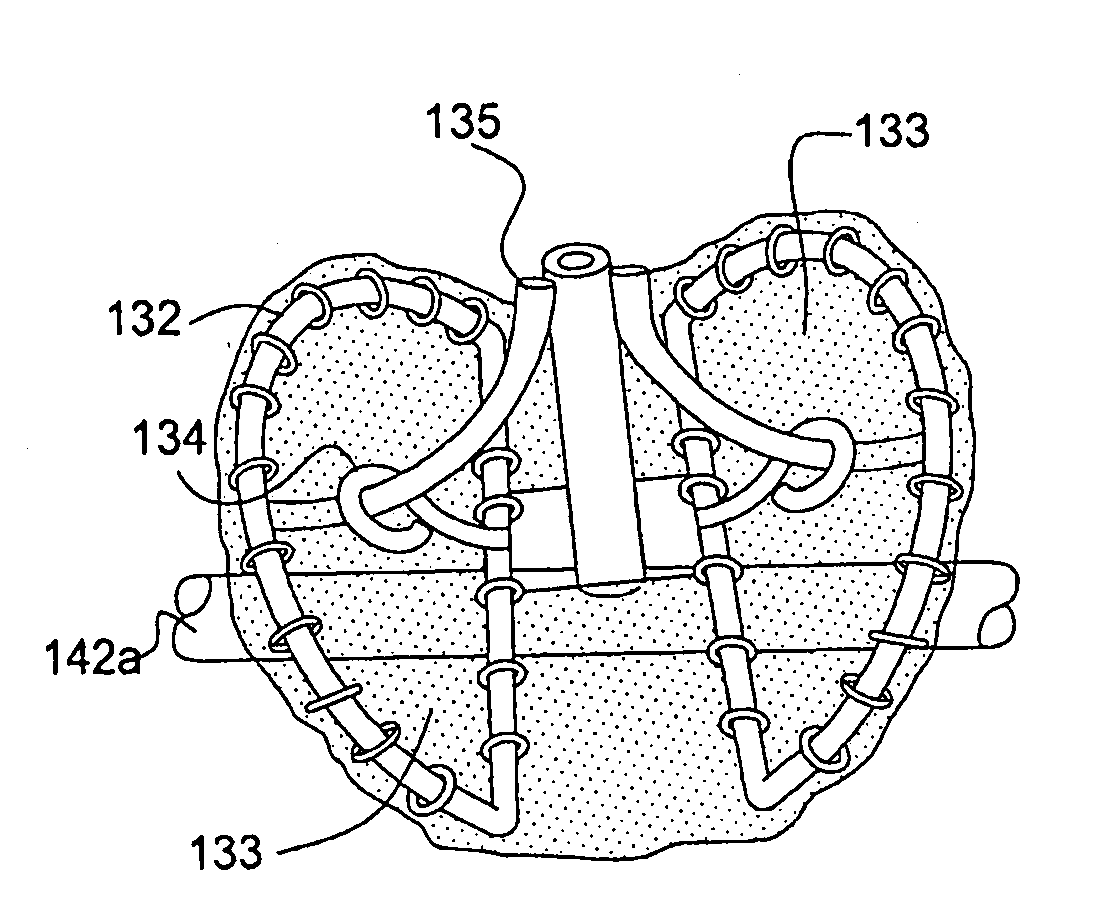
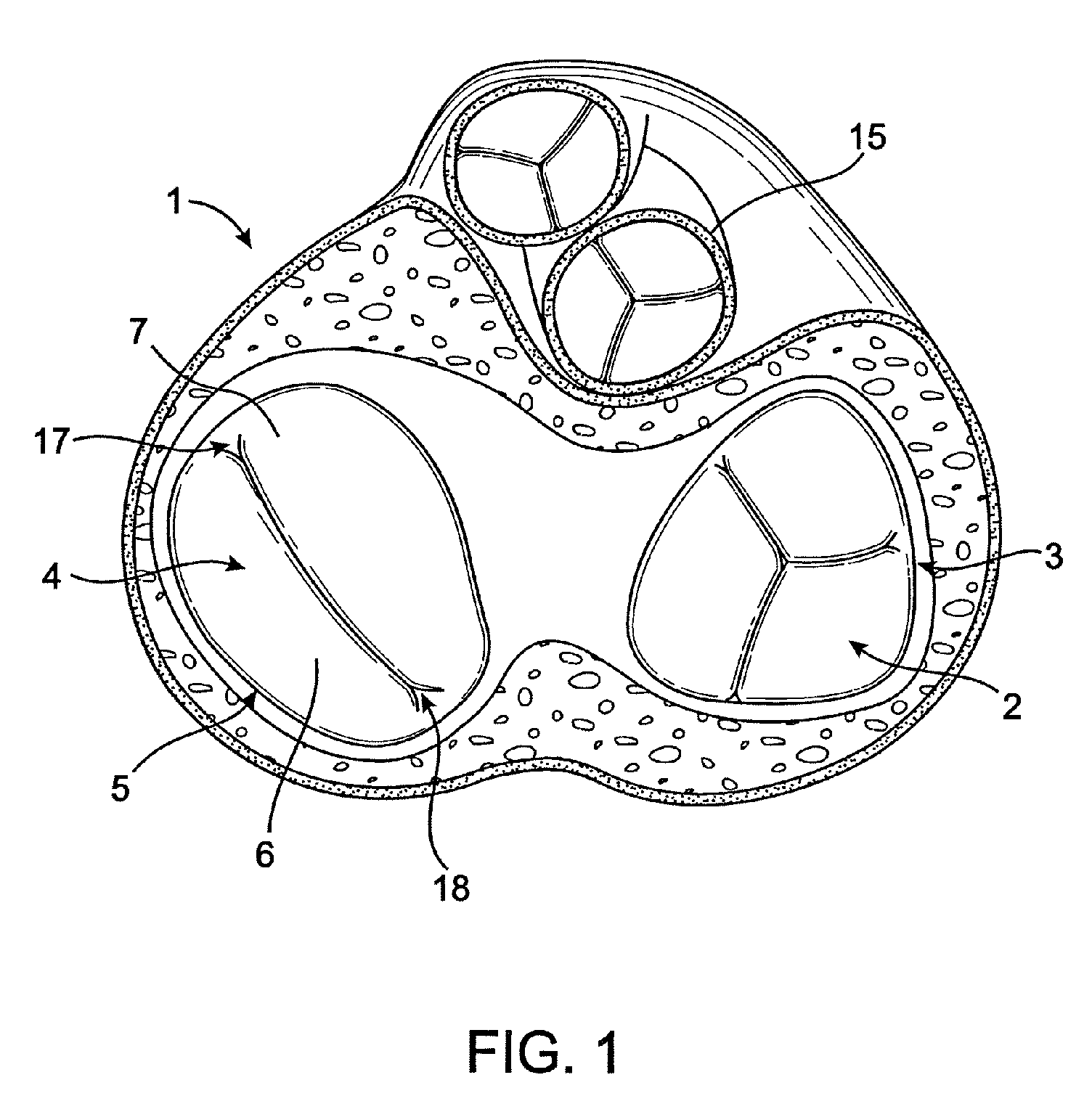
Collapsible valve having paravalvular leak protection
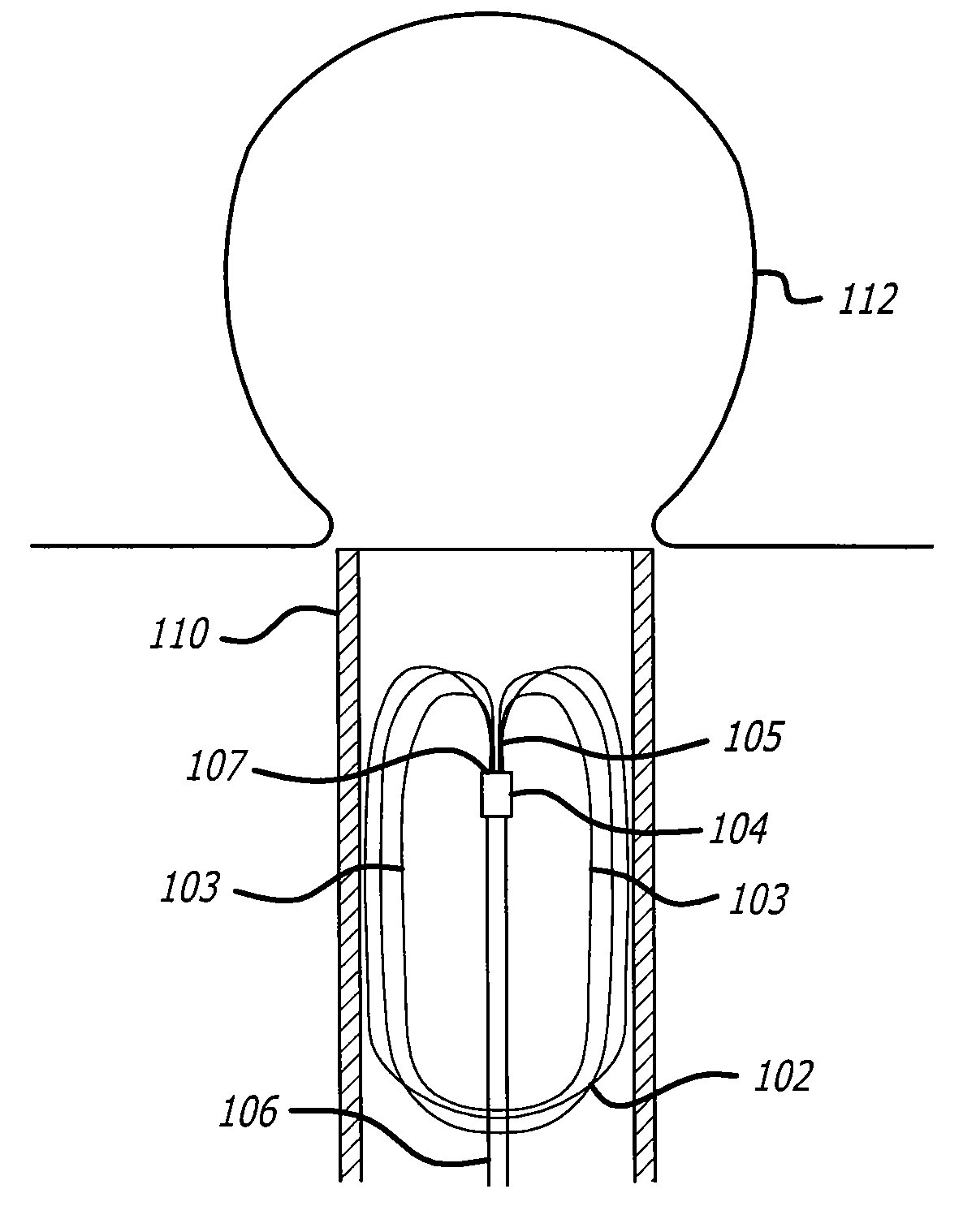
A heart valve assembly (100) includes a heart valve (116), a self-expandable and collapsible stent (112), and a sealing member (114). The stent (112) includes an inflow end (120) and an outflow end (122), and surrounds and supports the heart valve (116). The sealing member (114) is connected to the inflow end (120) of the stent (112) and extends around a periphery of the stent (112). The sealing member (114) is connected to the inflow end (120) of the stent (112), overlaps a portion of the heart valve (116), and extends around an outer periphery of the stent (112).
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com