Biocompatible crosslinked coating and crosslinkable coating polymer composition for forming such a coating
a crosslinkable coating and coating technology, applied in the field of implantable stents, can solve the problems of increasing the complexity of manufacturing and deployment, increasing the difficulty of braided stents, and increasing the complexity of the axial length between radially expanded and radially compressed conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
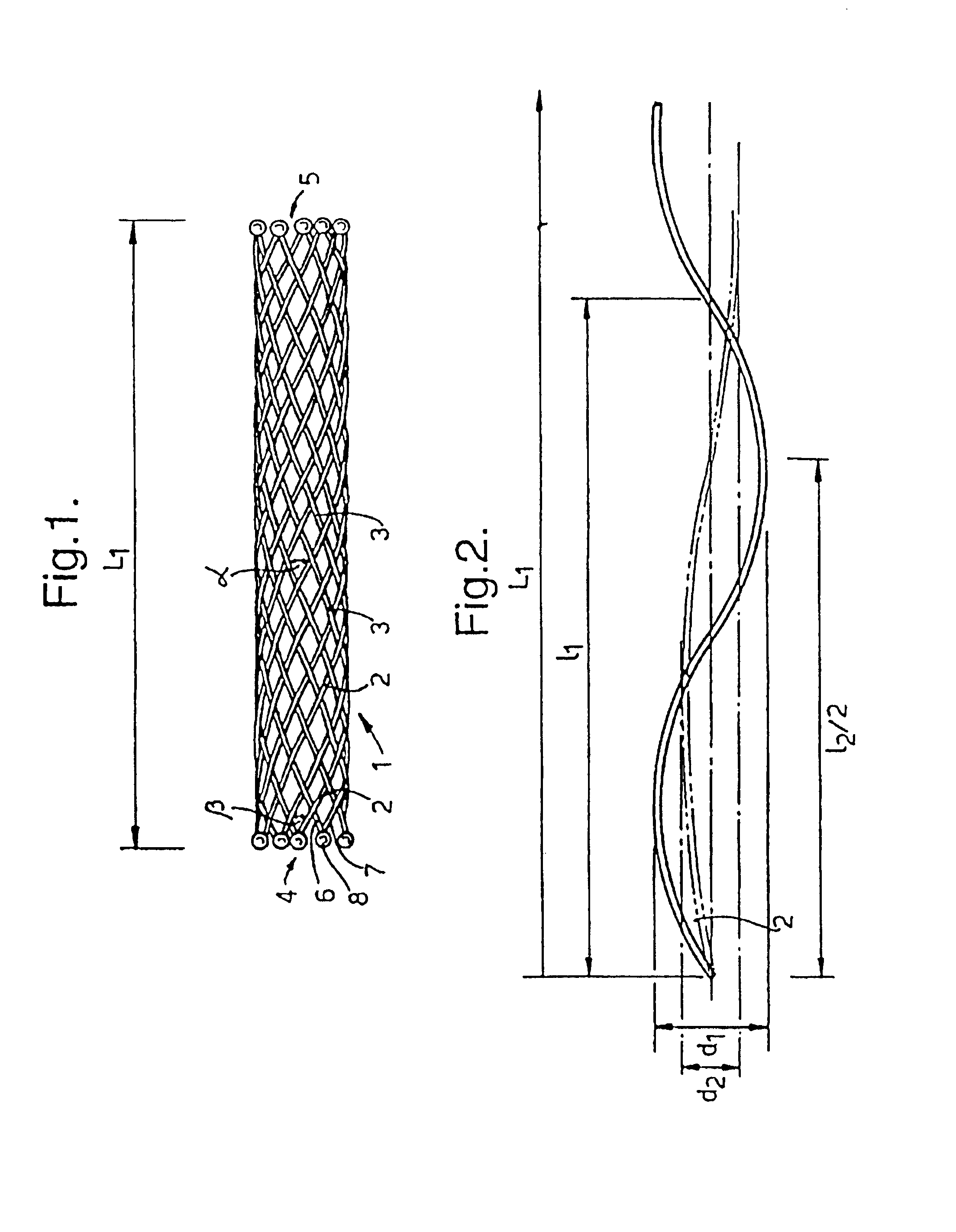
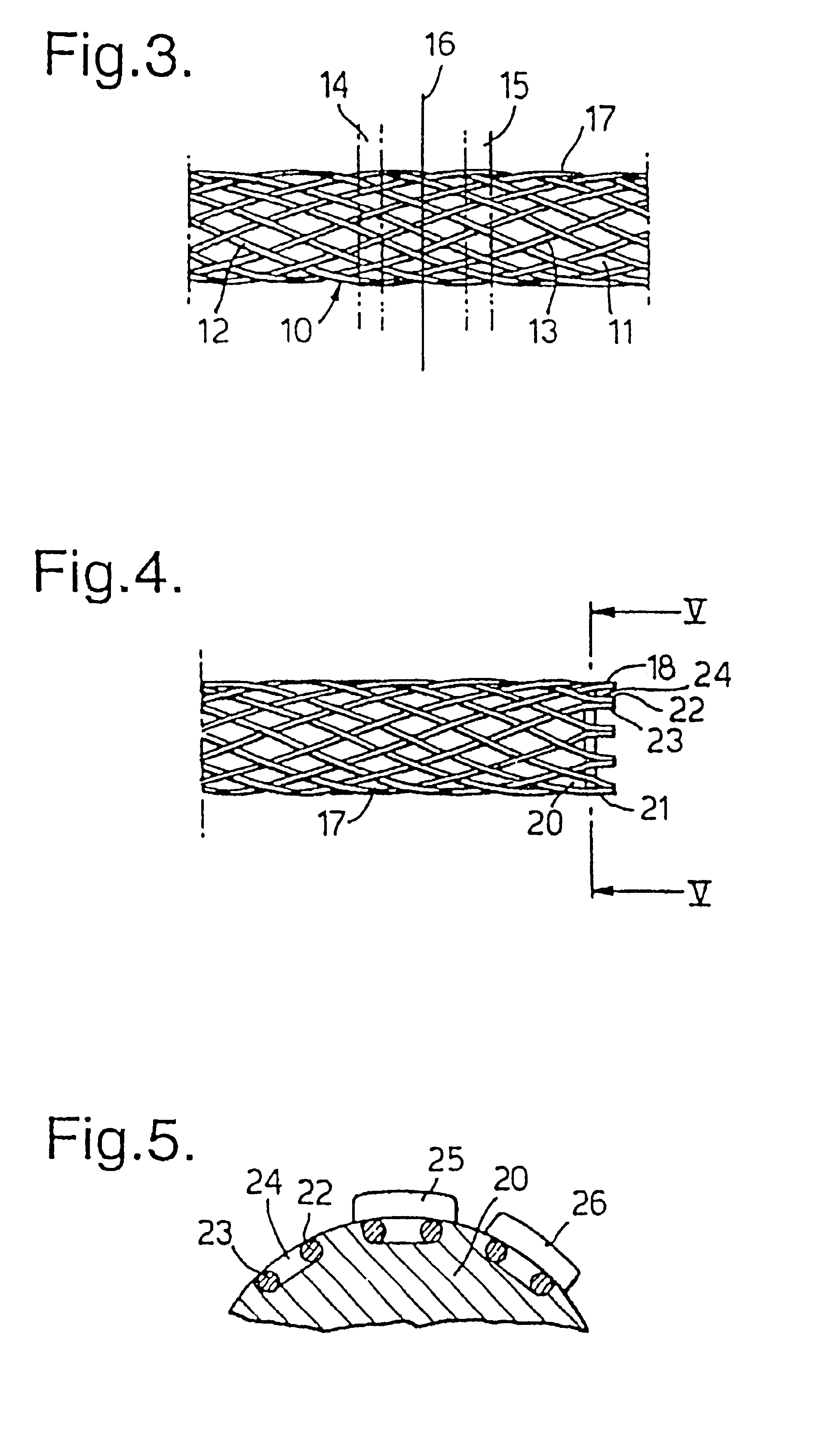
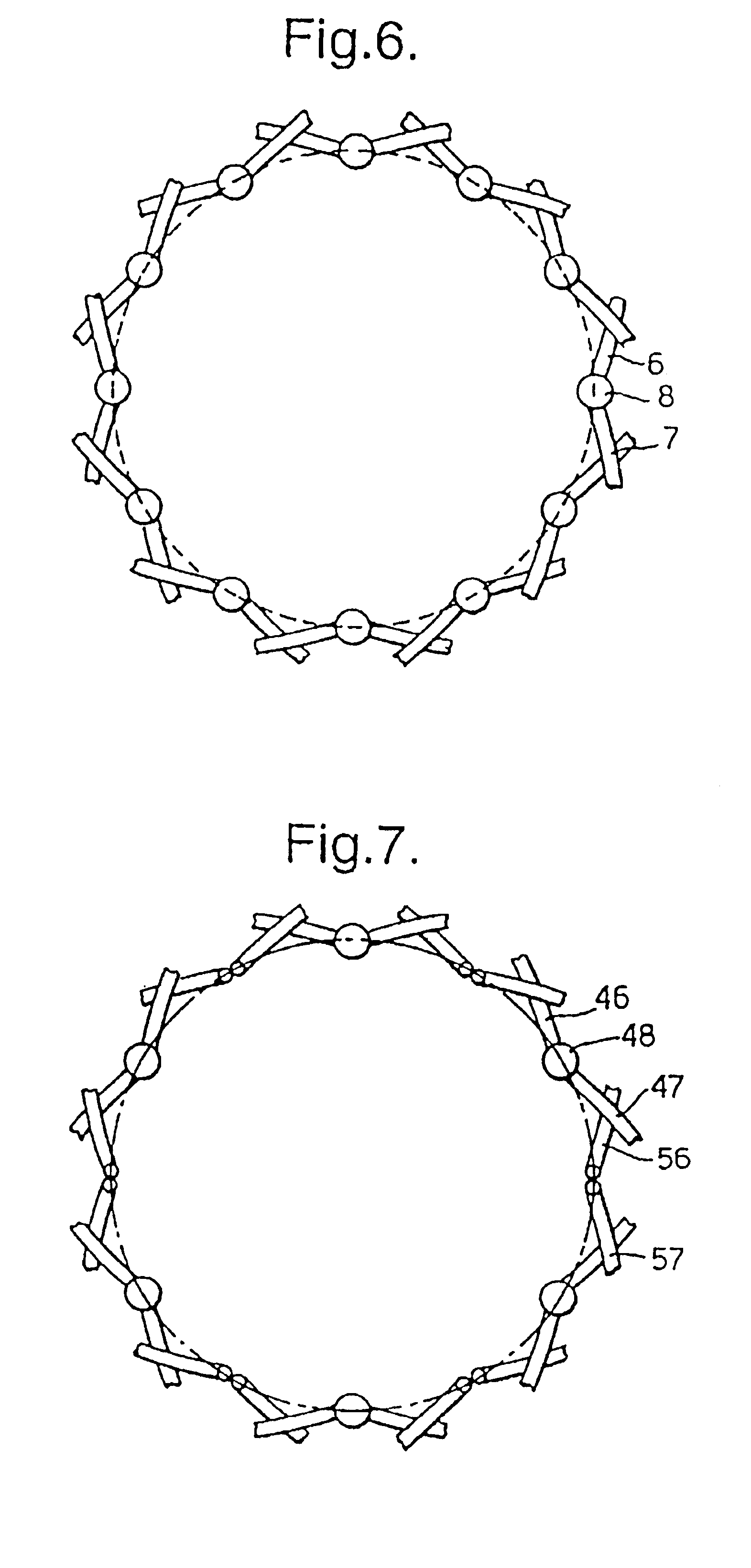
[0013]Although the ends of the filaments may be fixed together by other means, for instance by swaging as disclosed in GB-A-1,205,743, mentioned above, it is most convenient for the fixing to be by welding. Although the welding can be by resistance welding and / or by pressure, it is preferred for heat to be used, generally by plasma welding. Preferably the welding softens the metal such that it forms a globule before resolidifying to form a bead.
[0014]For some embodiments and applications it may be adequate to weld some but not all of the filament ends. For instance it may be convenient to weld every third pair of counter-rotating filaments at the end of one or both ends of the stent body. Preferably at least every other pair is welded at both ends, more preferably every pair at one, or preferably, both ends.
[0015]Preferably no filler wire is used in the welding although it may, for some purposes, be useful to include filler wire, for instance where the filler has different, usually ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com