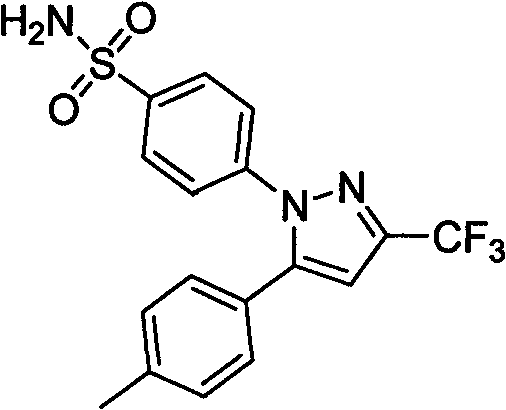
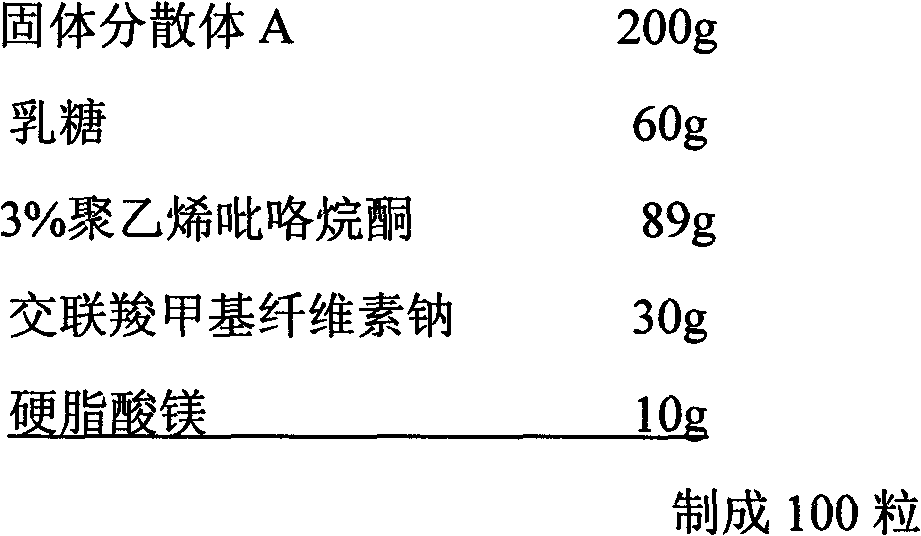
Celecoxib solid dispersion and preparation method and application thereof
A technology of solid dispersion and celecoxib, applied in the field of medicine, can solve the problems of difficult to form a uniform mixture, increased bioavailability, poor solubility, etc., and achieve the effects of improving bioavailability, simple preparation method and increasing absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation of Solid Dispersion A:
[0053] Heat 300g of poloxamer 188 in a water bath to 55°C to melt, and add 15g of celecoxib while stirring. Keep the mixture in a molten state, stir it well, pour it on a steel plate that has been pre-frozen at -25°C, solidify at -25°C for 3 minutes, dry it for 1 day, pulverize it, and then place it in a vacuum drying oven to fully dry to obtain Celecoxib Cloth Solid Dispersion A.
Embodiment 2
[0055] Preparation of Solid Dispersion B:
[0056] Heat 300g of poloxamer 188 in a water bath to 90°C to melt, and add 20g of celecoxib while stirring. Keep the mixture in a molten state, stir it well, pour it on a steel plate that has been pre-frozen at 0°C, solidify at a temperature below 0°C for 8 minutes, dry it for 2 days, crush it, and then place it in a vacuum drying oven to fully dry to obtain celecoxib solid Dispersion B.
Embodiment 3
[0058] Preparation of Solid Dispersion C:
[0059] Add 300g of poloxamer 188 into 30g of celecoxib in 900mL of acetone solution, heat to 55°C to melt, and stir thoroughly. Continue to heat the mixture to fully volatilize the acetone, pour it on a steel plate that has been pre-frozen at -15°C, solidify at -15°C for 3 minutes, dry it for 2 days, pulverize it, and then dry it fully in a vacuum drying oven to obtain celecoxib solid dispersion C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com