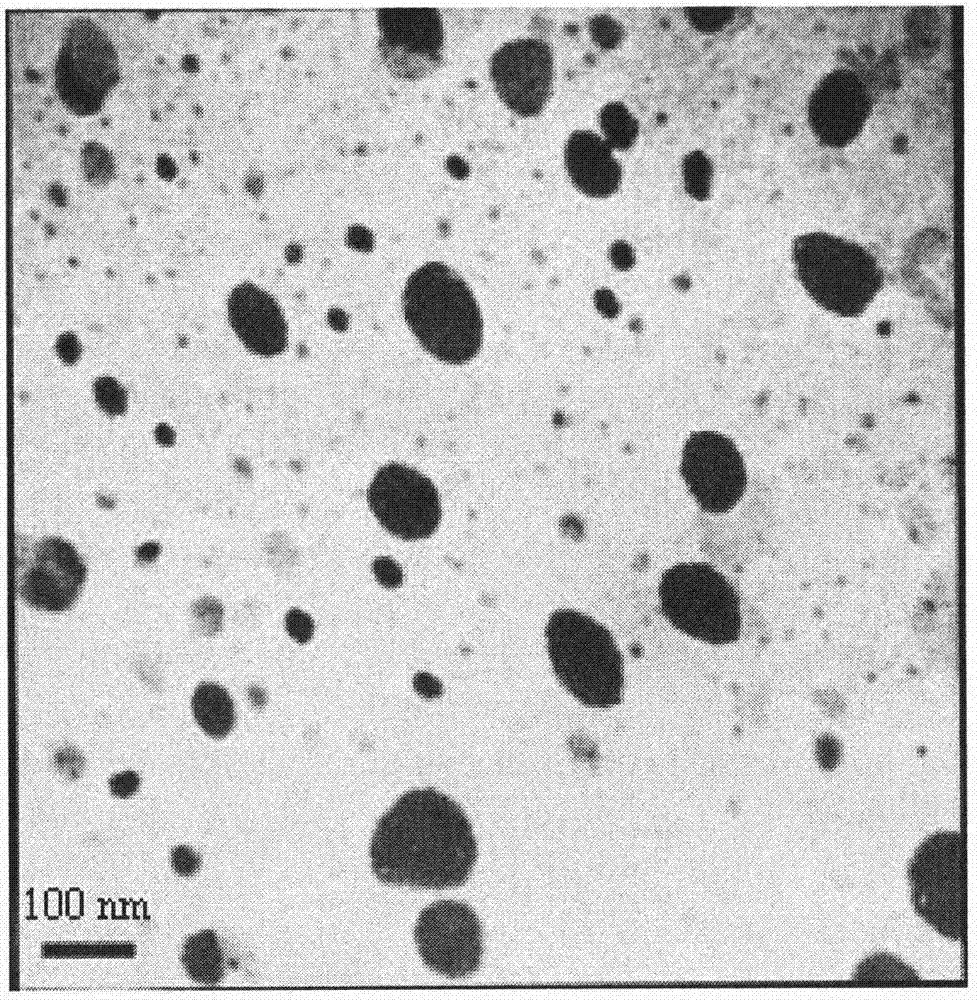
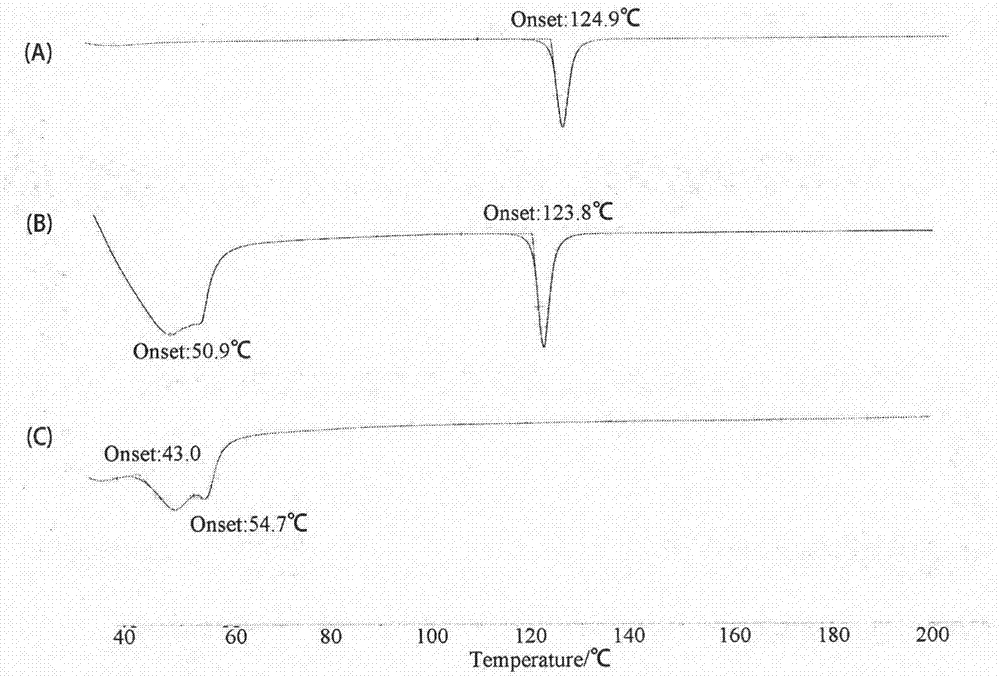
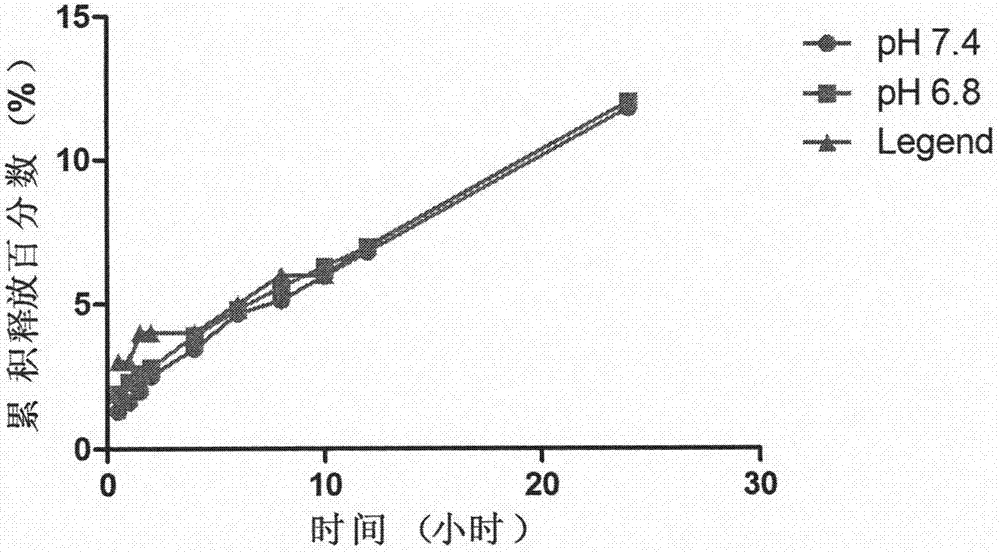
Nimodipine lipid nanoparticle and preparation technology thereof
A nimodipine lipid and lipid nanoparticle technology, which is applied to medical preparations containing no active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve the problem of long hydration time, easy leakage of drugs, and reduced Production efficiency and other issues, to achieve the effect of avoiding organic solvent residues, uniform distribution of active ingredients, and avoiding direct contact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Prescription composition:
[0029]
[0030] Preparation Process:
[0031] (1) The mixed lipids of glyceryl distearate and propylene glycol dicaprate according to a certain mass ratio are melted in a water bath at 80° C. for subsequent use;
[0032] (2) Dissolve the prescribed amount of nimodipine in an appropriate amount of ethanol in a water bath at 80°C, then slowly add it to the melted mixed lipids, then heat and evaporate the solvent to form an oil phase, and set aside;
[0033] (3) Evenly disperse the prescribed amount of Tween 80 in an appropriate amount of pure water under the condition of a water bath at 80° C. to form a water phase for later use;
[0034] (4) adding the oil phase in step (2) to the water phase in step (3), while high-speed shearing for a period of time to form colostrum;
[0035] (5) The colostrum obtained in the step (4) is quickly added to a high-pressure homogenizer and circulated several times, and cooled to room temperature to obtain ...
Embodiment 2
[0038] Prescription composition:
[0039]
[0040] Preparation Process:
[0041] (1) place the glyceryl distearate of prescription quantity under 80 ℃ of water bath conditions and melt, standby;
[0042] (2) Dissolve the prescribed amount of nimodipine in an appropriate amount of ethanol in a water bath at 80°C, then slowly add it to the melted solid lipid, then heat and evaporate the solvent to form an oil phase, and set aside;
[0043] (3) Evenly disperse the prescribed amount of Tween 80 in an appropriate amount of pure water under the condition of a water bath at 80° C. to form a water phase for later use;
[0044] (4) adding the oil phase in step (2) to the water phase in step (3), while high-speed shearing for a period of time to form colostrum;
[0045] (5) Add the colostrum obtained in step (4) into a high-pressure homogenizer and circulate it several times, and cool to room temperature to obtain the nimodipine lipid nanoparticle colloidal dispersion.
[0046] The ...
Embodiment 3
[0048] Prescription composition:
[0049]
[0050] Preparation Process:
[0051] (1) The glyceryl monostearate and propylene glycol dicaprate mixed lipids of the prescribed amount are melted in a water bath at 80° C. according to a certain mass ratio, and set aside;
[0052] (2) Dissolve the prescribed amount of nimodipine in an appropriate amount of ethanol in a water bath at 80°C, then slowly add it to the melted mixed lipids, then heat and evaporate the solvent to form an oil phase, and set aside;
[0053] (3) Evenly disperse the prescribed amount of Tween 80 in an appropriate amount of pure water under the condition of a water bath at 80° C. to form a water phase for later use;
[0054] (4) adding the oil phase in step (2) to the water phase in step (3), while high-speed shearing for a period of time to form colostrum;
[0055] (5) Add the colostrum obtained in step (4) into a high-pressure homogenizer and circulate it several times, and cool to room temperature to ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com