Quercetin glycoside composition and method of preparing the same
A technology of quercetin glycosides and compositions, which is applied in the field of new compositions and can solve the problems of limited utilization and poor solubility in water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0149] III. The preparation method of quercetin glycoside composition
[0150] The quercetin glycoside composition of the present invention with strong oral absorbability can be used as a raw material for enzymatic treatment of isoquercitrin, after reducing the general formula (2):
[0151] Chemical formula 12
[0152]
[0153] (In the formula, Glc represents a glucose residue, and n represents an integer of 4 or more.)
[0154] The indicated amount of quercetin glycoside [IQC-G(4≤)] is prepared in a process whose total amount is 20 mol% or less.
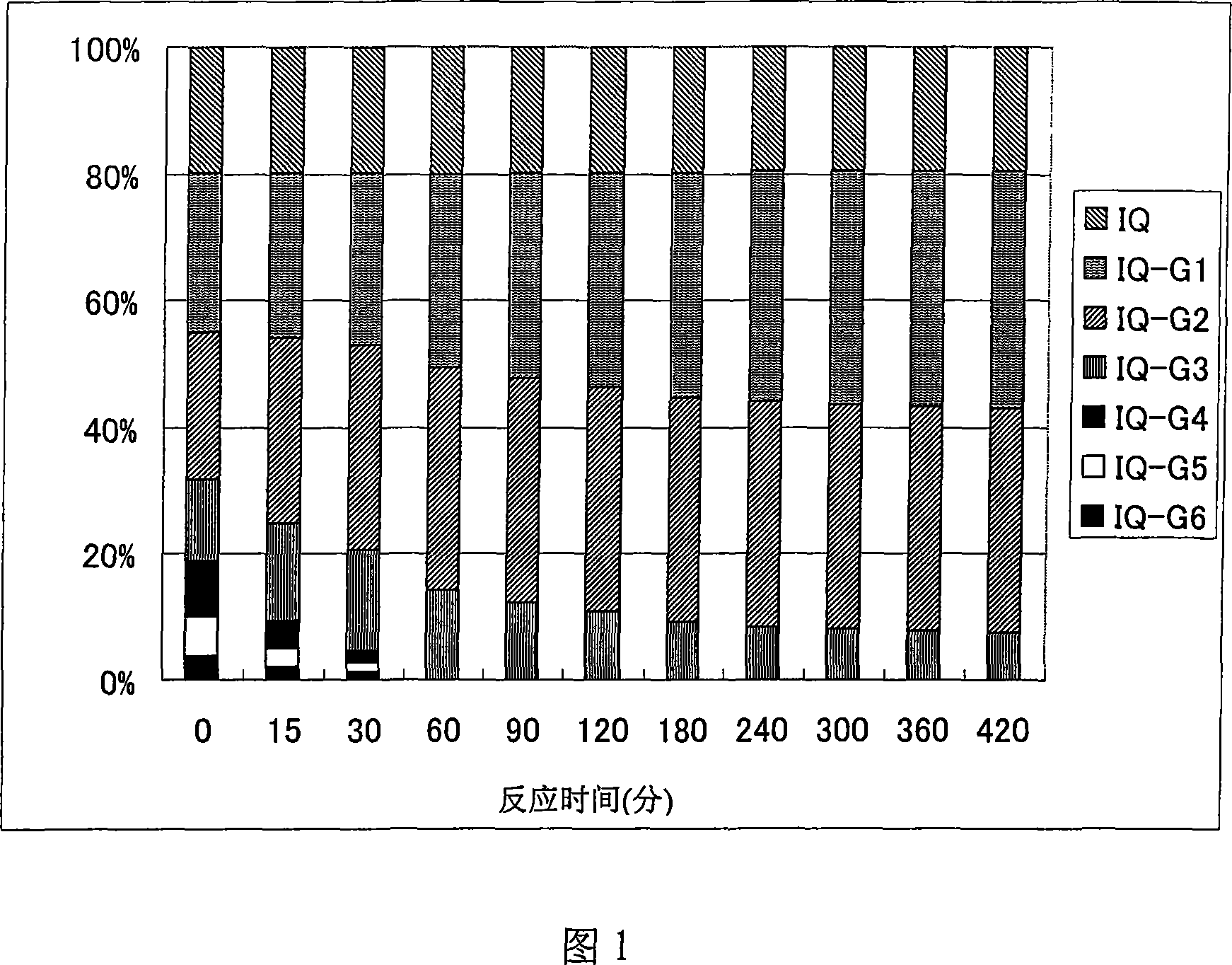
[0155] Here, regardless of the method for reducing the amount of IQC-G (4≤), for example, the method of separating and removing IQC-G (4≤) from enzyme-treated isoquercitrin, decomposing enzyme-treated isoquercitrin containing Any method such as the IQC-G(4≤) method can be used. A method in which the enzyme-treated isoquercitrin is treated with amylase may be preferred.
[0156] The amylase used here is not particularly limi...
preparation example 1
[0212] Reference Preparation Example 1 Preparation of Enzymatically Treated Isoquercitrin
[0213] (1) Preparation of isoquercitrin
[0214] After immersing 250 g of flower buds of Sophora japonica, a leguminous plant, in 2500 mL of hot water (above 95° C.) for 2 hours, the filtrate was taken as the “first extract”. In addition, the filtered residue is soaked and extracted with hot water to obtain a "second extract". The above first and second extracts were combined, cooled to below 30° C., and the precipitated components were filtered out, washed with water, recrystallized, and dried to obtain 22.8 g of rutin with a purity of 95% or higher.
[0215] 20 g of this rutin was dispersed in 400 mL of water, and the pH was adjusted to 4.9 using a pH regulator. 0.12 g of naringinase [Amano Enzyme Inc., trade name "Naringinase 'AMANO'", 3,000 U / g] was added thereto to start the reaction, which was kept at 72°C for 24 Hour. Thereafter, the reaction liquid was cooled to 20° C....
preparation example 2
[0252] Preparation example 2 Preparation of Isoquercitrin G(1-3) Components and Isoquercitrin G(3-6) Components
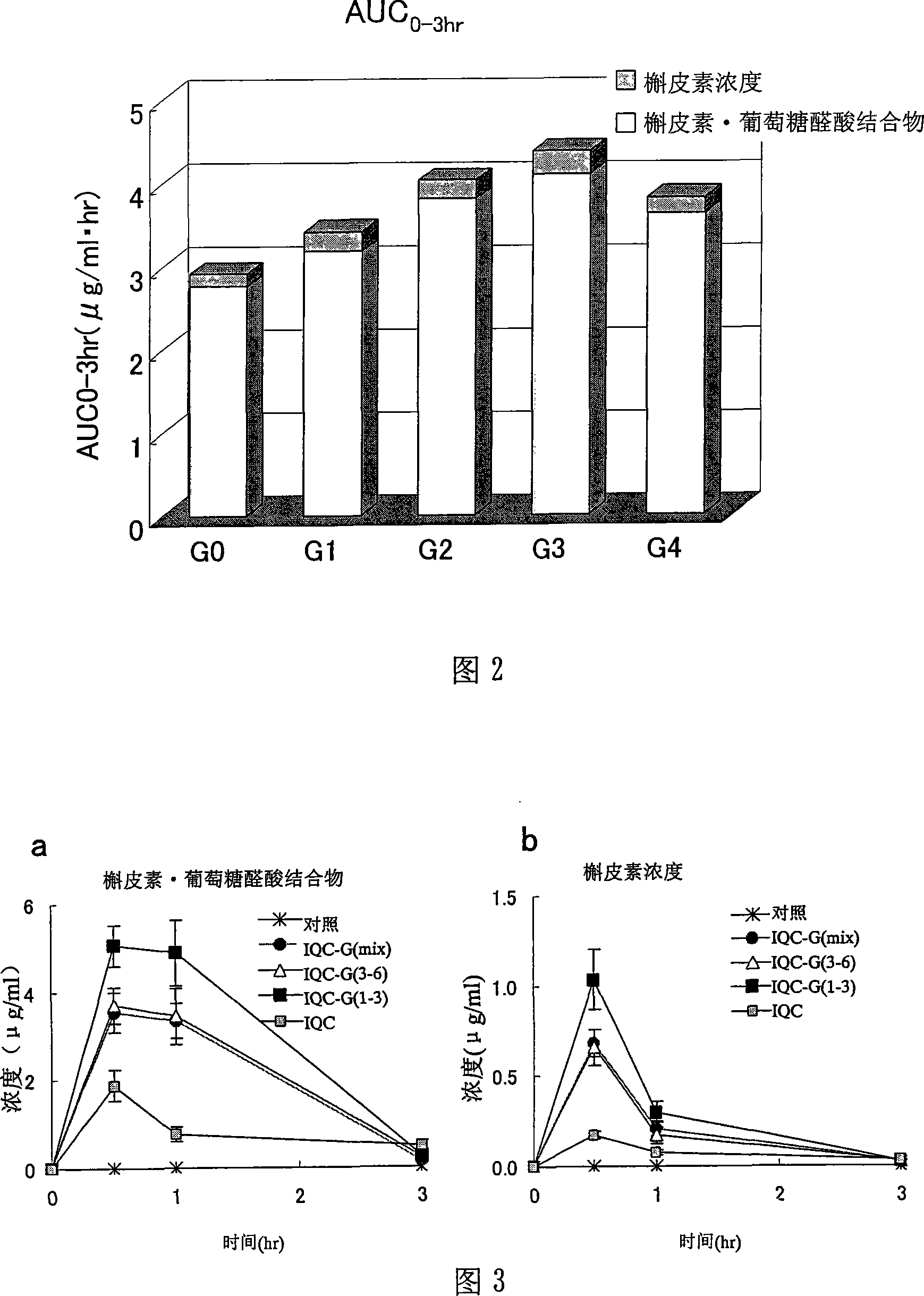
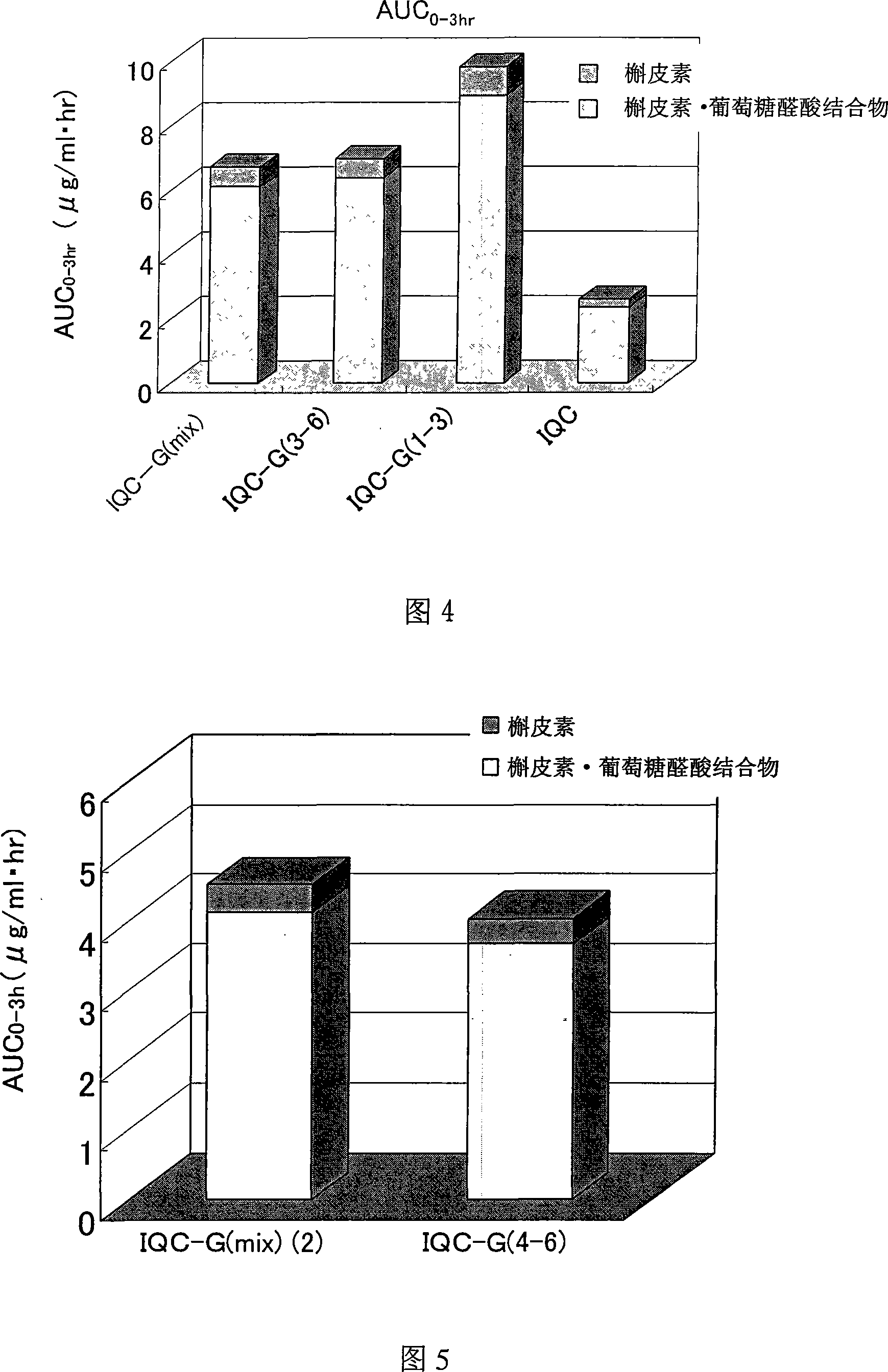
[0253] 0.65 g of the enzyme-treated isoquercitrin [IQC-G(mix)] obtained in Reference Preparation Example 1 was dissolved in aqueous methanol, and gel filtration chromatography was performed using a gel filtration resin (Sephadex LH-20: Amersham Bioscience K.K.) analyze. After the effluent was divided into a certain amount, it was analyzed by HPLC using the conditions described in the above-mentioned reference preparation example 1, and it was divided into G3, 4 G4, and 5 glucose bound by α1, 4 glycosidic bonds on the rich IQC. Combined G5 and 6 combined G6 components (hereinafter referred to as "isoquercitrin G(3-6) component" or "IQC-G(3-6)" component), and enriched on IQC A component of G1, 2 G2 and 3 G3 combined with α1,4 glycosidic bonds (hereinafter referred to as "isoquercitrin G(1-3) component" or "IQC-G (1-3) Components") of 3 components. Thereafter, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com