Ornithine aspartate effervescent tablets and preparing process thereof
A technology of ornithine aspartic acid and effervescent tablets, which is applied to medical preparations containing active ingredients, drug delivery, digestive system, etc., to achieve high bioavailability, rapid onset, and fast dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
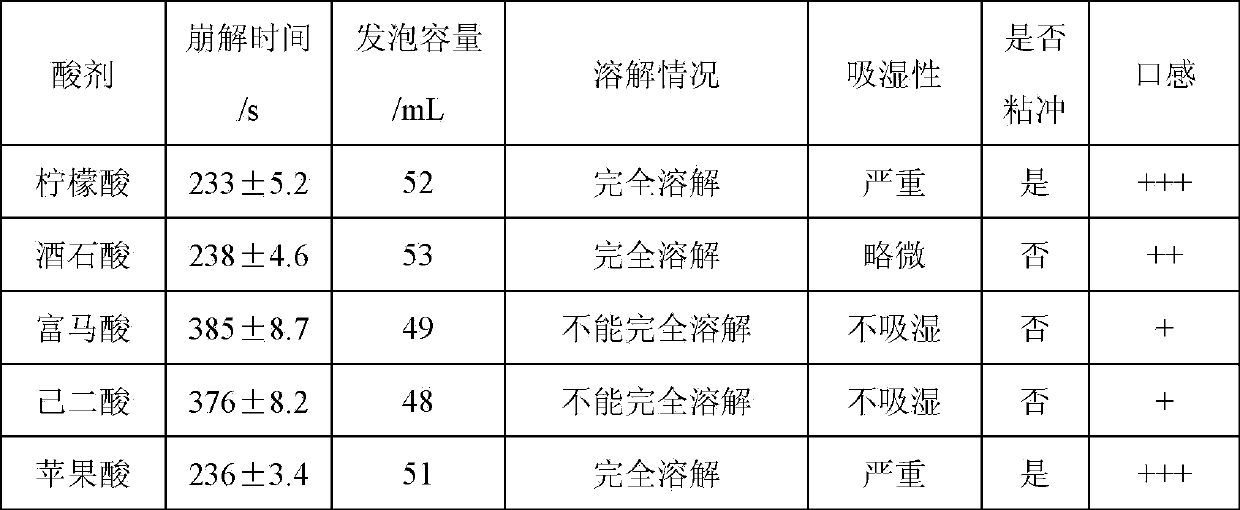
[0032] Embodiment 1 uses different organic acids to prepare ornithine-aspartic acid effervescent tablets
[0033] prescription
[0034] Citric acid (288 g), tartaric acid (338 g), fumaric acid (261 g), adipic acid (329 g) and malic acid (302 g) were used as acid agents, respectively. Other ingredients in the prescription are as follows:
[0035] Ornithine Aspartate
1000g
Povidone K30
45g
210g
stevia
0.75
0.75
[0036] polyethylene glycol 6000
150
production
1000 pieces
[0037] method
[0038] Using polyethylene glycol encapsulation-wet granulation process:
[0039] 1) Take polyethylene glycol 6000 and mix evenly with sodium bicarbonate, heat in a water bath at 70°C for 20 minutes to melt, then pulverize after cooling, and pass through an 80-mesh sieve.
[0040] 2) Take ornithine aspartate, add tartaric acid, mix evenly, use povidone K30 ethanol...
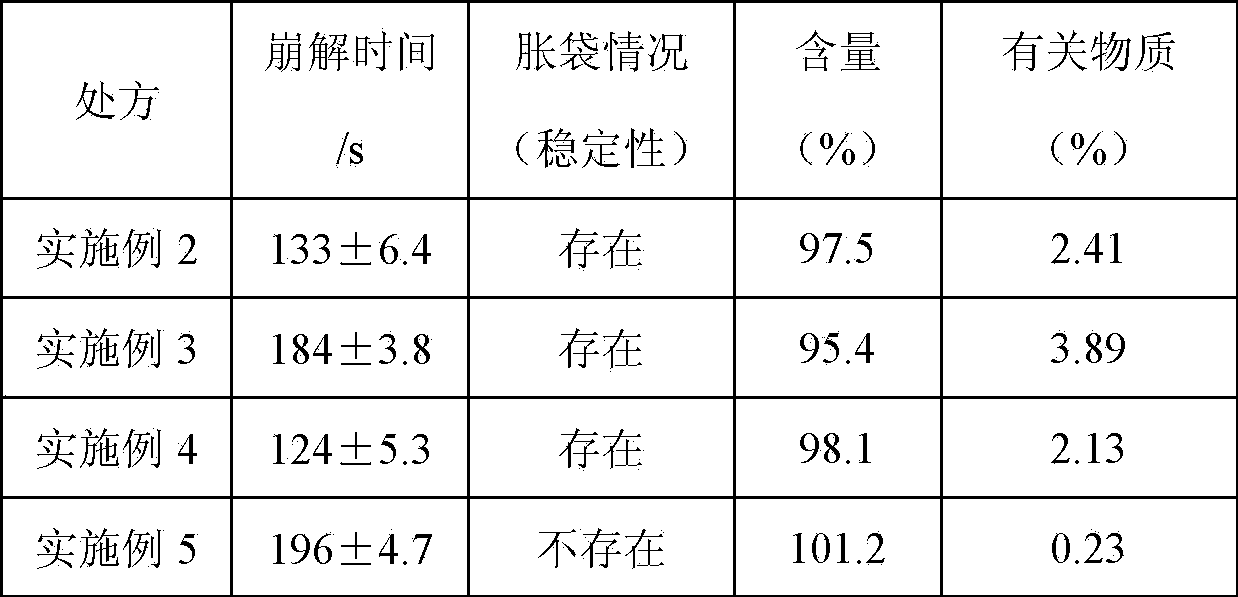
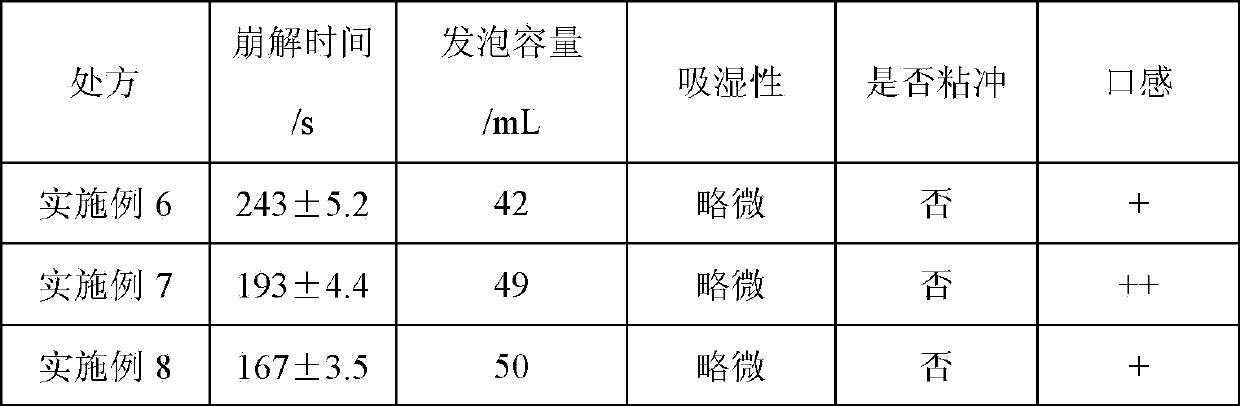
Embodiment 2-5
[0047] Embodiment 2-5: the screening of preparation process
Embodiment 2
[0048] Embodiment 2 dry granulation process
[0049] prescription
[0050] Ornithine Aspartate
1000g
Copovidone S630
45g
338g
210g
stevia
0.75
0.75
polyethylene glycol 6000
30
production
1000 pieces
[0051] method
[0052] Using dry granulation process:
[0053] 1) Take ornithine aspartate, copovidone S630, tartaric acid, sodium bicarbonate, and mix well.
[0054] 2) Rolling into large pieces and crushing to obtain granules.
[0055] 3) Add stevioside, orange essence and polyethylene glycol 6000, mix well, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com