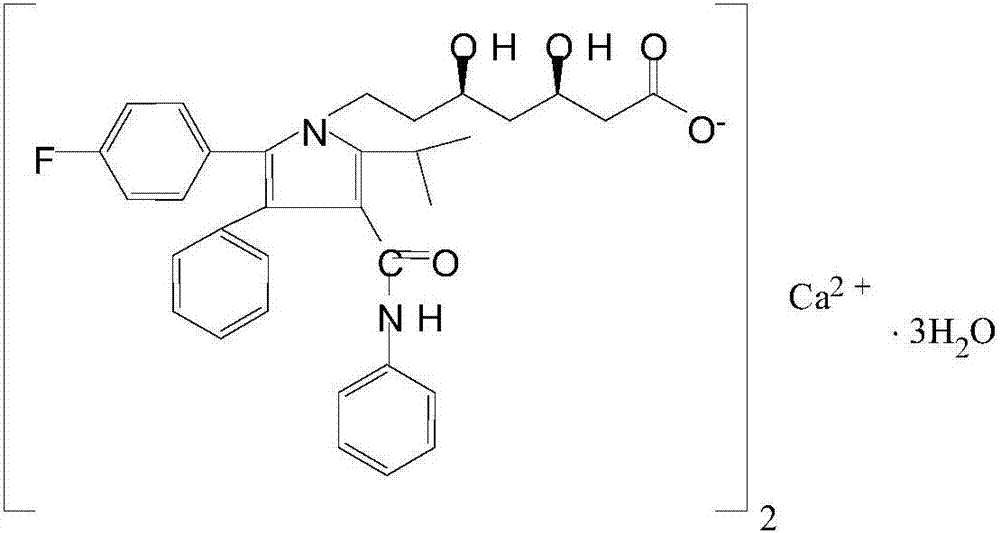
Tablet comprising atorvastatin calcium alkaline solid dispersoid and preparation method of tablet
A technology of atorvastatin calcium and solid dispersion, which is applied in the direction of medical preparations of non-active ingredients, active ingredients of heterocyclic compounds, and pill delivery, which can solve problems such as increased substances, complicated processes, and poor stability, and achieve High dissolution rate, high bioavailability and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
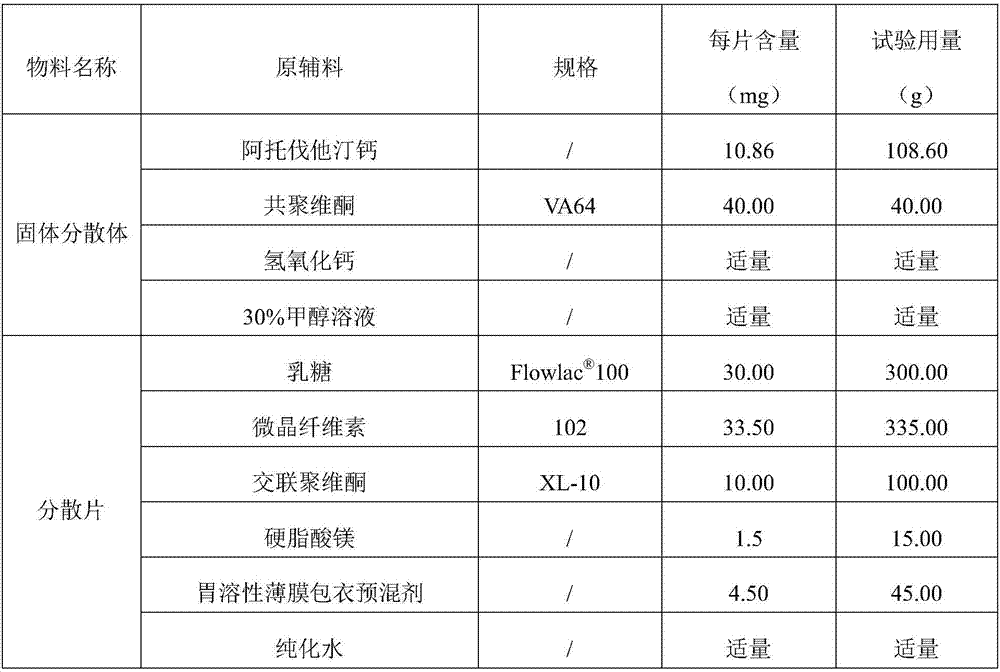
[0036] Atorvastatin calcium tablet and preparation process
[0037]
[0038] Preparation Process
[0039] (1) Dissolve the prescribed amount of atorvastatin calcium in 30% methanol solution, add the prescribed amount of copovidone to dissolve, add calcium hydroxide to adjust the pH to 9.0, remove the solvent by fluidized bed drying, and obtain a solid dispersion Body spare.
[0040] (2) After mixing the prescription amount of atorvastatin calcium solid dispersion, lactose, microcrystalline cellulose, and crospovidone for 10 minutes, add the prescription amount of magnesium stearate, mix for 2 minutes, and press the theoretical tablet weight Tablets were carried out to obtain plain tablets.
[0041] (3 Use a high-efficiency coating machine, put the atorvastatin calcium tablets into the coating pan, control the tablet bed temperature to about 50°C, and the flow rate to be 40g / min, and coat.
Embodiment 2
[0043] Atorvastatin calcium tablet and preparation process
[0044]
[0045]
[0046] Preparation Process
[0047] (1) Dissolve the prescribed amount of atorvastatin calcium in 30% methanol solution, add the prescribed amount of copovidone to dissolve, add calcium hydroxide to adjust the pH to 10.0, remove the solvent by fluidized bed drying, and obtain a solid dispersion Body spare.
[0048] (2) After mixing the prescription amount of atorvastatin calcium solid dispersion, lactose, microcrystalline cellulose, and crospovidone for 10 minutes, add the prescription amount of magnesium stearate, mix for 2 minutes, and press the theoretical tablet weight Tablets were carried out to obtain plain tablets.
[0049] (3) Put the atorvastatin calcium tablet into the coating pan with a high-efficiency coating machine, control the tablet bed temperature to about 50°C, and the flow rate to 40g / min, and coat.
Embodiment 3
[0051] Atorvastatin calcium tablet and preparation process
[0052]
[0053]
[0054] Preparation Process
[0055] (1) Dissolve the prescribed amount of atorvastatin calcium in 30% methanol solution, add the prescribed amount of copovidone to dissolve, add calcium hydroxide to adjust the pH to 11.0, remove the solvent by fluidized bed drying, and obtain a solid dispersion Body spare.
[0056] (2) After mixing the prescription amount of atorvastatin calcium solid dispersion, lactose, microcrystalline cellulose, and crospovidone for 10 minutes, add the prescription amount of magnesium stearate, mix for 2 minutes, and press the theoretical tablet weight Tablets were carried out to obtain plain tablets.
[0057] (3) Put the atorvastatin calcium tablet into the coating pan with a high-efficiency coating machine, control the tablet bed temperature to about 50°C, and the flow rate to 40g / min, and coat.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com