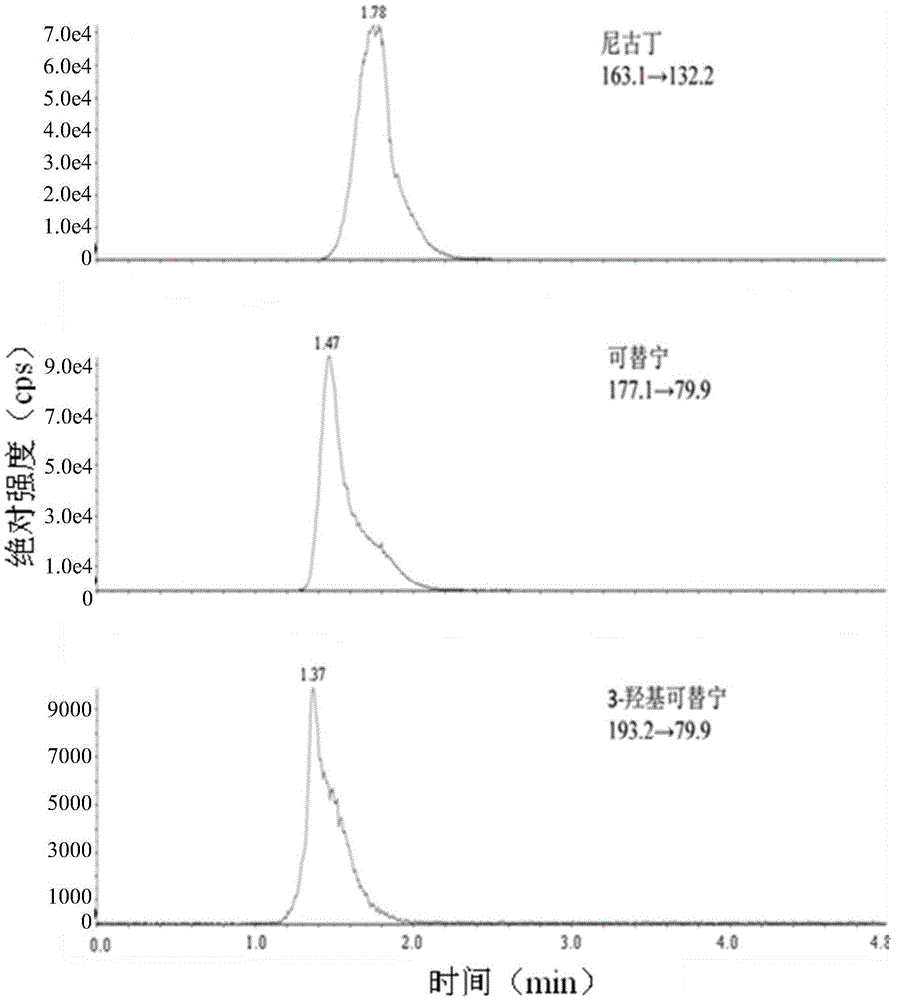
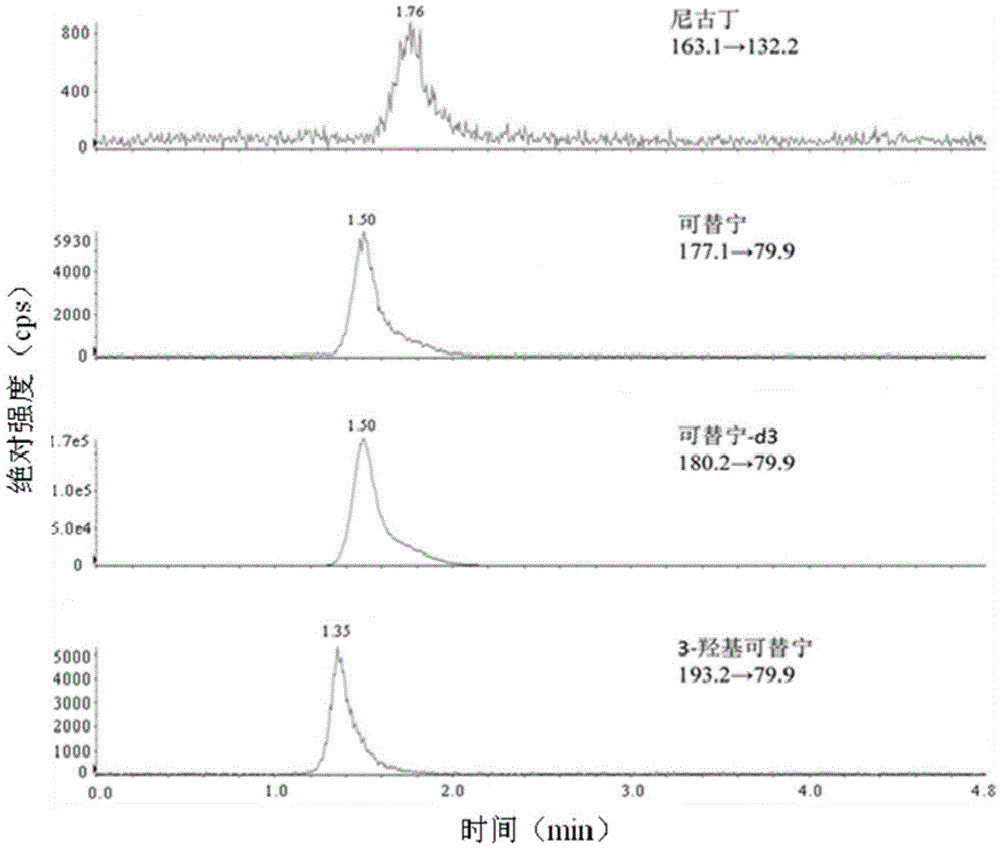
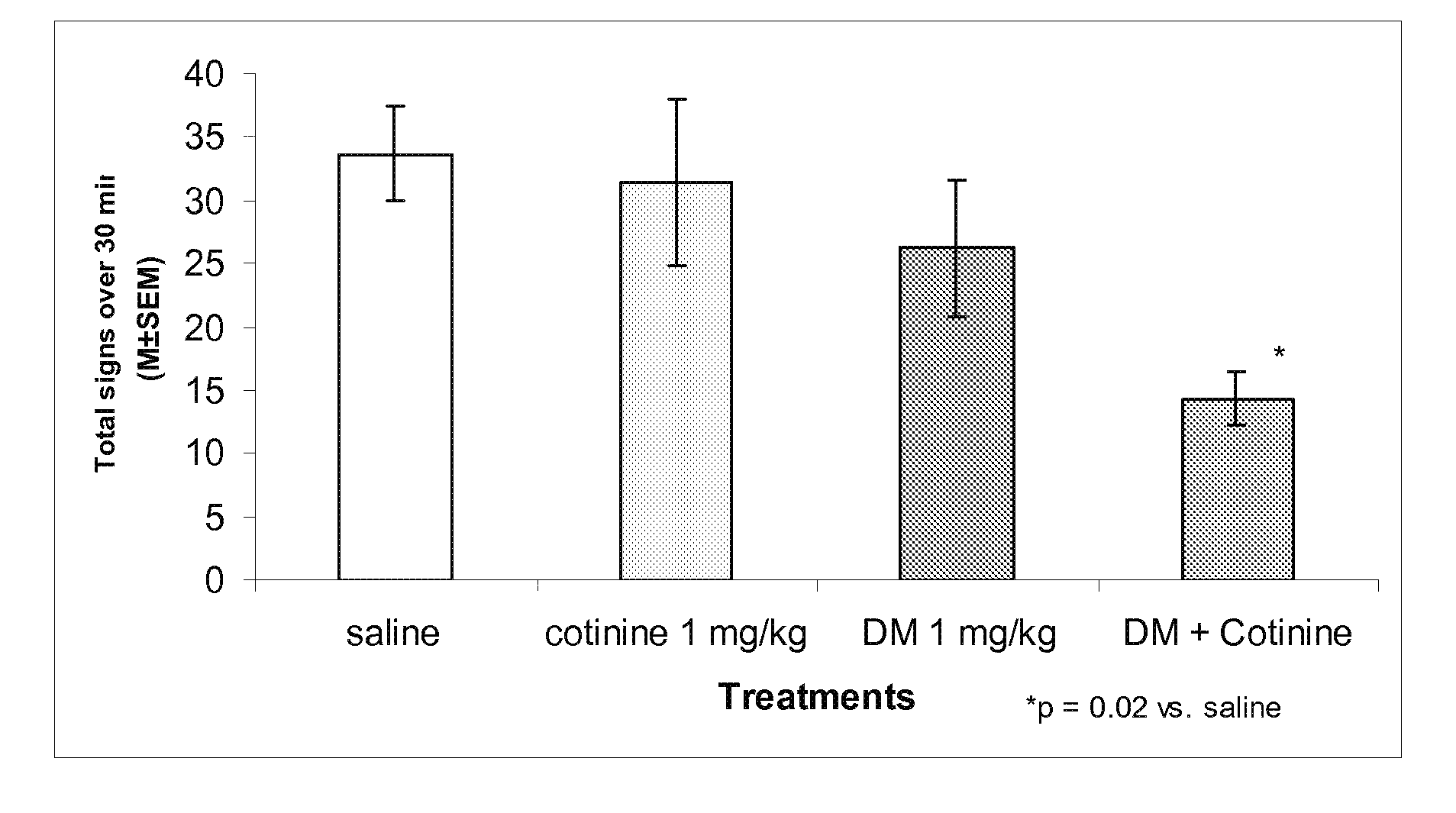Patents
Literature
35 results about "Urine cotinine level" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
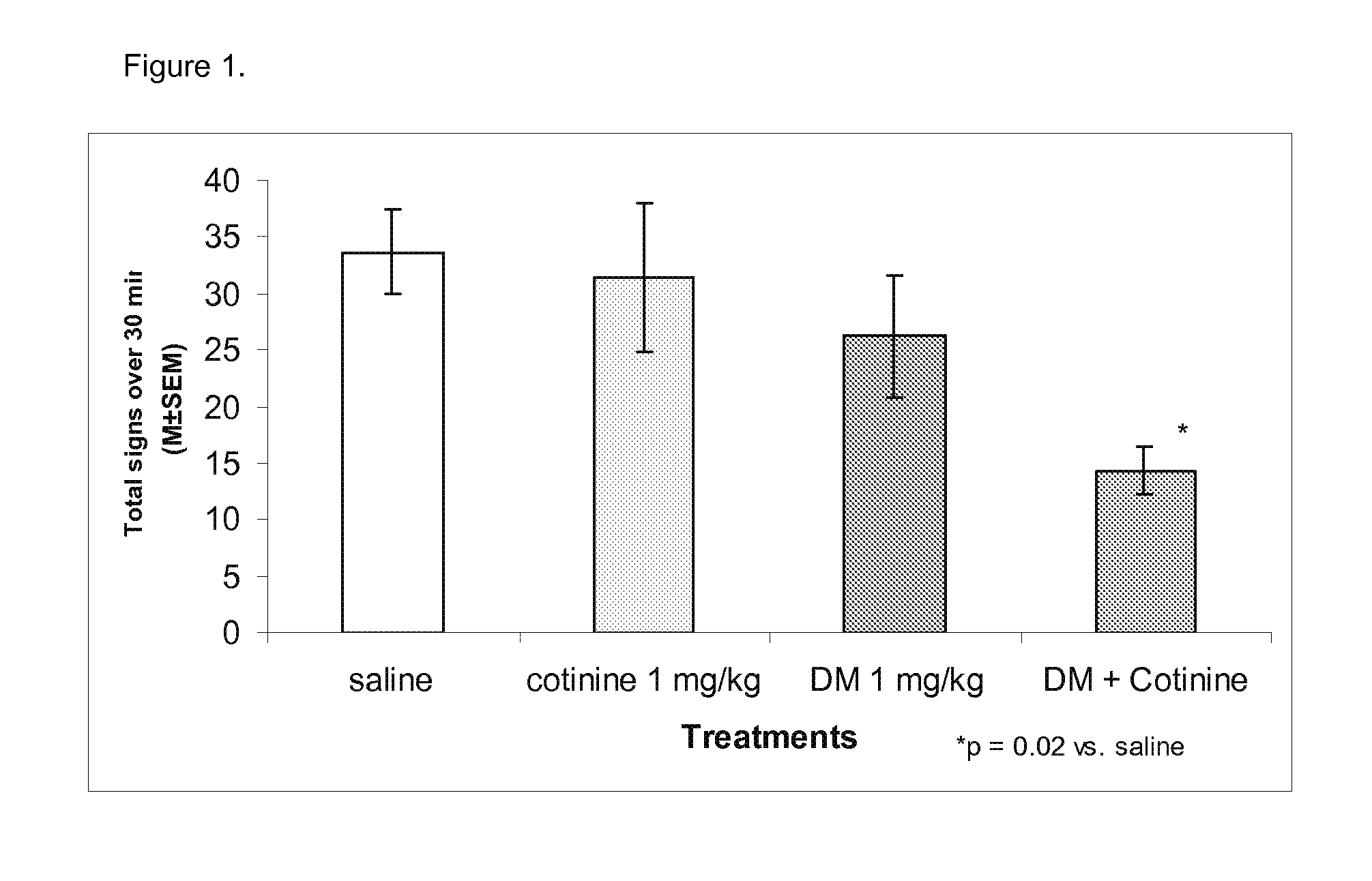
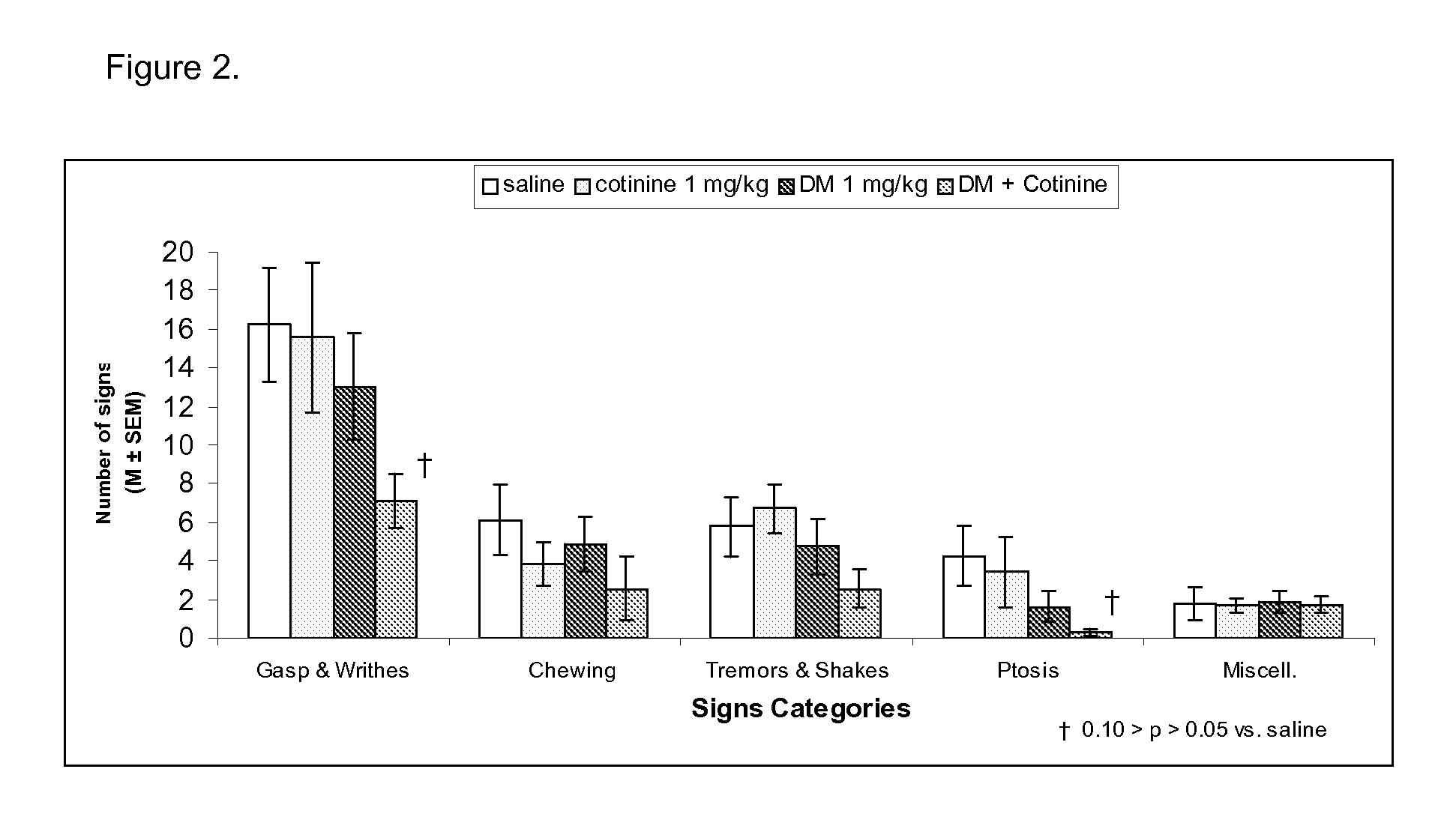
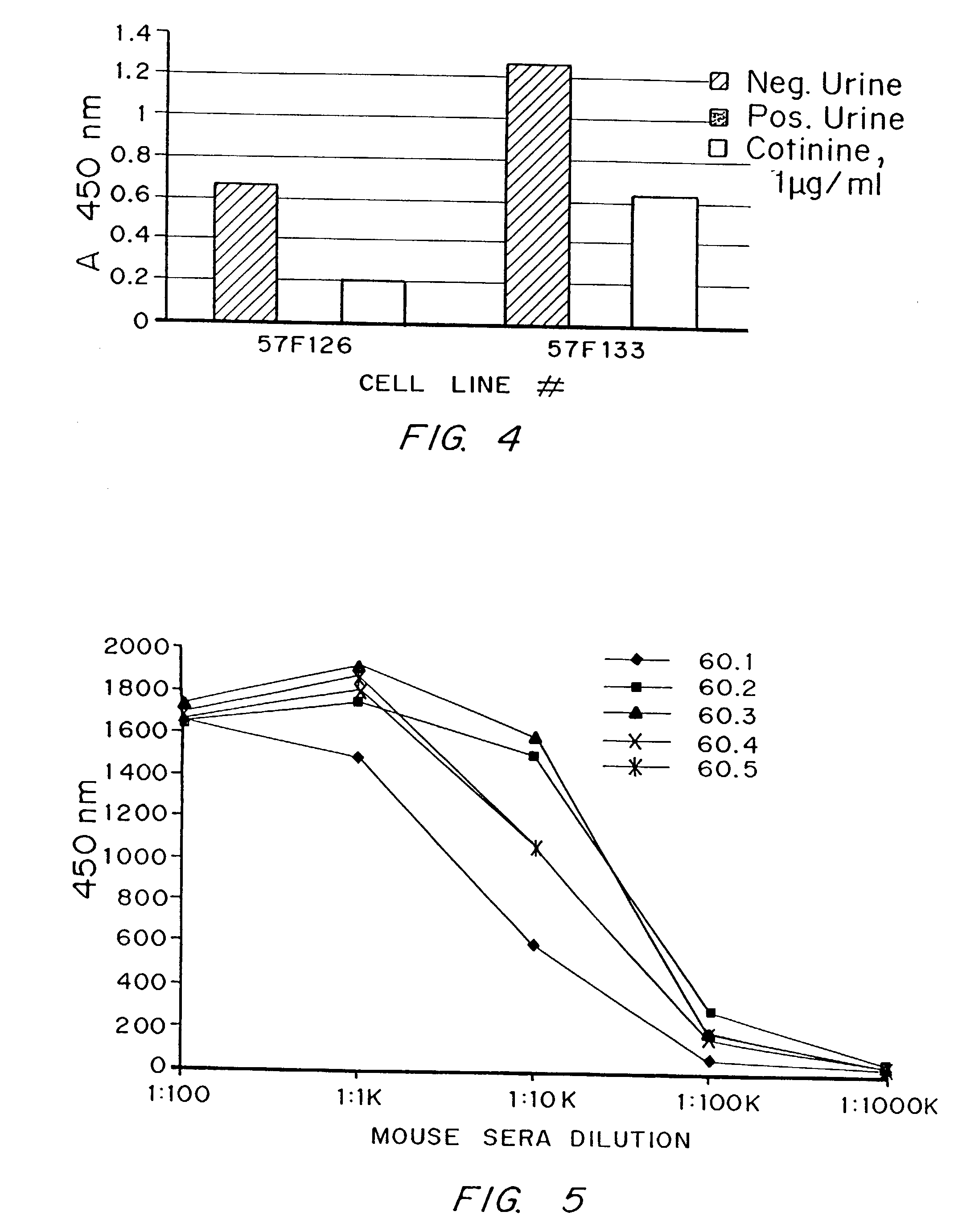
Urine cotinine concentrations average four to six times higher than those in blood or saliva, making urine a more sensitive matrix to detect low-concentration exposure. Cotinine levels <10 ng/mL are considered to be consistent with no active smoking.
Compositions for Reducing Nicotine Withdrawal Symptoms and/or Tobacco Usage
InactiveUS20100040679A1Reducing nicotine withdrawal symptomRelieve symptomsBiocideNervous disorderDrug withdrawal symptomsMetabolite
Compositions useful for treating an individual with nicotine dependence comprising a combination of an α3β4 nicotinic receptor antagonist and a nicotine metabolite are disclosed. More particularly, compositions comprising cotinine or a pharmaceutically acceptable salt thereof are disclosed. Methods of alleviating nicotine withdrawal symptoms and / or tobacco usage by administration of these compositions are also disclosed.
Owner:SMITHKLINE BECKMAN CORP
Multi-Portion Intra-Oral Dosage Form With Organoleptic Properties
InactiveUS20100247586A1Efficient and effectiveCosmetic preparationsNervous disorderActive agentUrine cotinine level
A multi portion intra-oral dosage form has at least one pharmaceutically active agent or health promoting agent with at least one portion comprises a component for creating a noticeable organoleptic sensation. The sensory markers or signals are conceptual aids for a subject using the dosage form where the organoleptic sensation is such that it facilitates the subject to identify a portion and differentiate between different portions. Also contemplated are a dosage form, a method and a system for delivering active agents, such as nicotine and / or metabolites thereof, such as cotinine, nicotine N′-oxide, nomicotine, (S)-nicotine-N-β-glucuronide and mixtures, isomers, salts and complexes thereof as well as use and production of said dosage forms.
Owner:MCNEIL AB
Multi portion intra-oral dosage form and use thereof
InactiveUS20100124560A1Efficient and effectiveReduce impulseBiocideCosmetic preparationsNornicotineActive agent
The present invention relates to a multi portion intra-oral dosage form where at least one portion is rapidly disintegrating and at least one portion is slowly disintegrating, whereby the disintegration time for the slowest disintegrating portion is at least two times longer than for the most rapidly disintegrating portion. Of certain interest is use of sensory markers / signals as conceptual aids for the subject.Also contemplated are a method and a system for delivering active agents, such as nicotine and / or metabolites thereof, such as cotinine, nicotine N′-oxide, nornicotine, (S)-nicotine-N-β-glucuronide and mixtures, isomers, salts and complexes thereof as well as use and production of said formulations.
Owner:MCNEIL AB
Integrated packaging holder device for immunochromatographic assays in flow-through or dipstick formats
InactiveUS6087185ABioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteUrine cotinine level
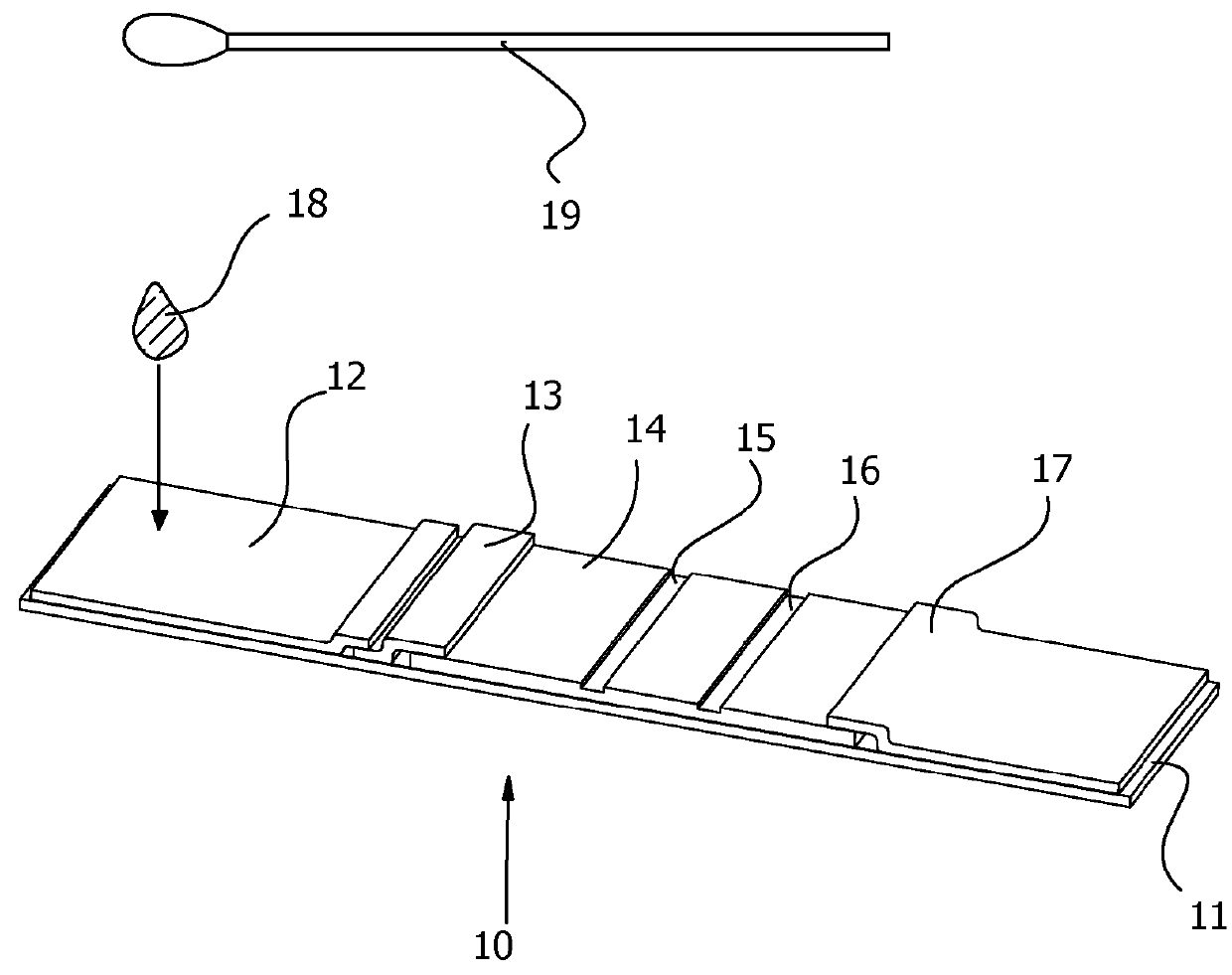
The prevent invention relates to an integrated package-holder assay devices for detecting the presence of analyte in a sample. The device serves the dual roles of supporting and protecting an immunochromatographic assay. The device is compatible with any immunochromatographic assay format. The assay can be performed in a single apparatus for use in a laboratory or a field setting. In a specific example, the assay device is a nylon membrane formatted for an immunochromatographic assay for cotinine sealed between transparent adhesive tape and a stiff plastic strip. White tape placed over the plastic strip defined a window for observing the assay results.
Owner:SEREX
Cotinine neutralizing antibody
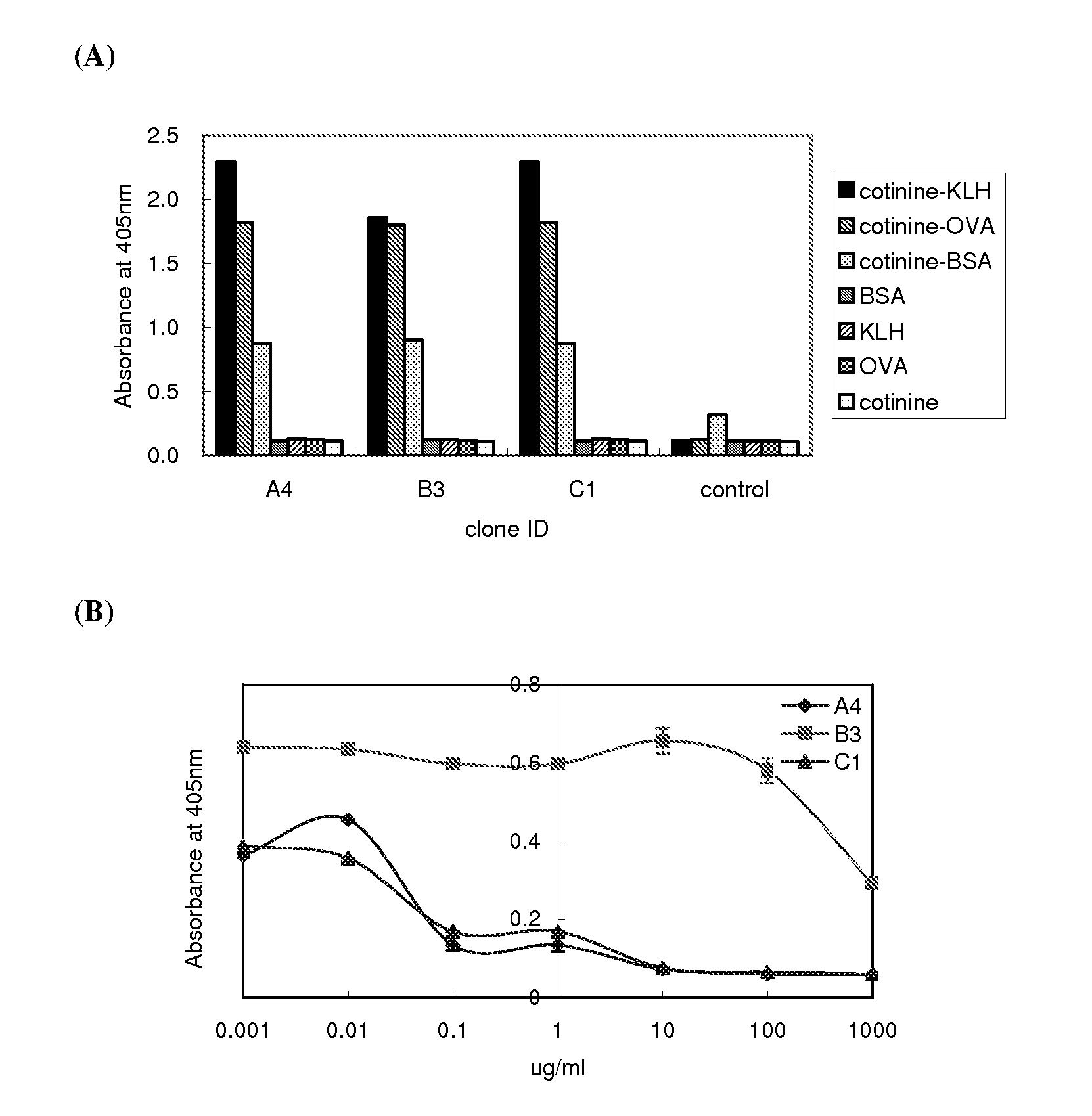
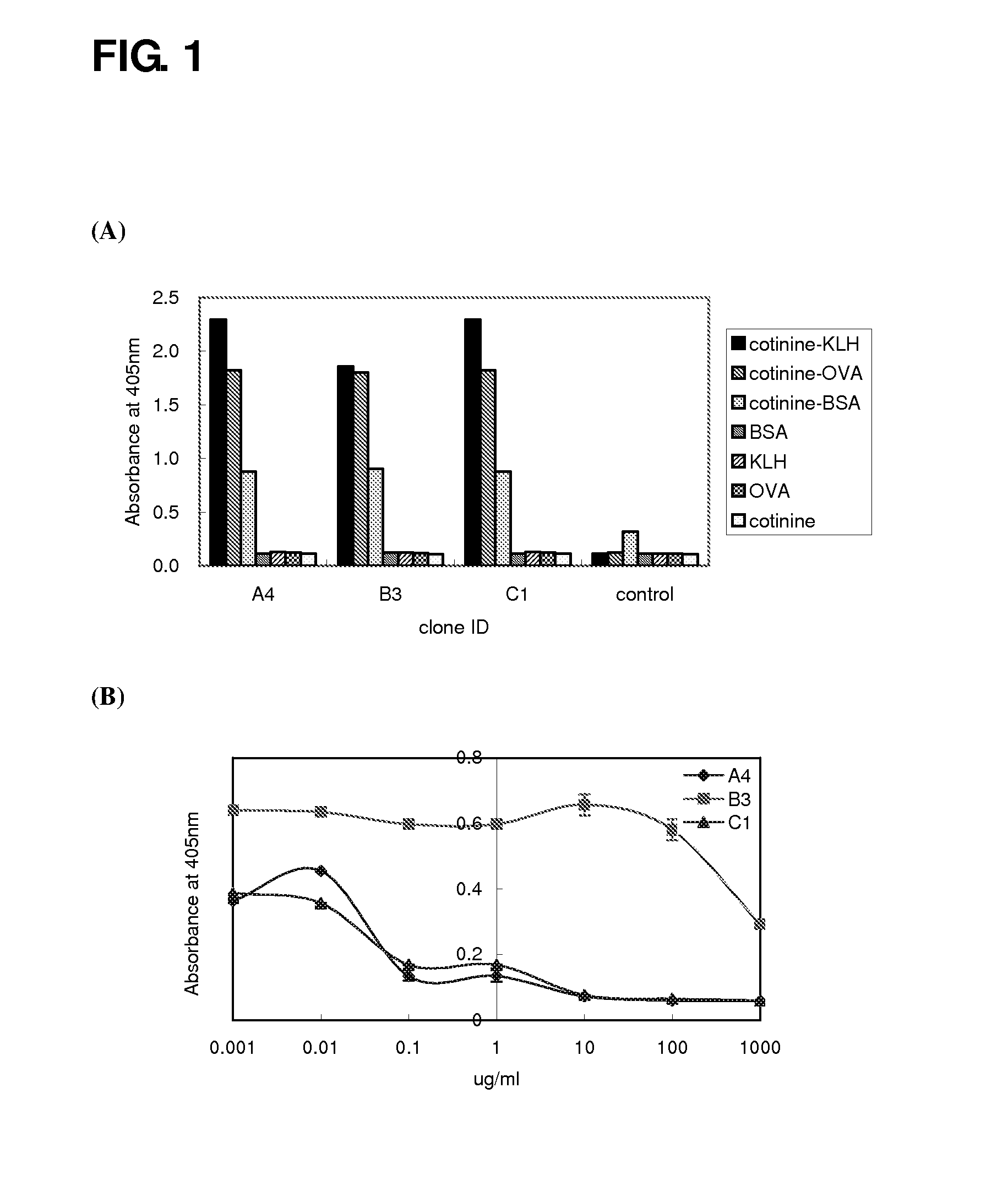
ActiveUS20080226650A1Inhibition is effectiveReduce the binding forceNervous disorderSugar derivativesMonoclonal antibodyUrine cotinine level
Owner:NAT CANCER CENT
Multi portion intra-oral dosage form and use thereof
The present invention relates to a multi portion intra-oral dosage form where at least one portion is rapidly disintegrating and at least one portion is slowly disintegrating, whereby the disintegration time for the slowest disintegrating portion is at least two times longer than for the most rapidly disintegrating portion. Of certain interest is use of sensory markers / signals as conceptual aids for the subject.,Also contemplated are a method and a system for delivering active agents, such as,nicotine and / or metabolites thereof, such as cotinine, nicotine N'-oxide, nornicotine, (S)-nicotine-N-ss-glucuronide and mixtures, isomers, salts and complexes thereof as well as use and production of said formulations.
Owner:MCNEIL AB
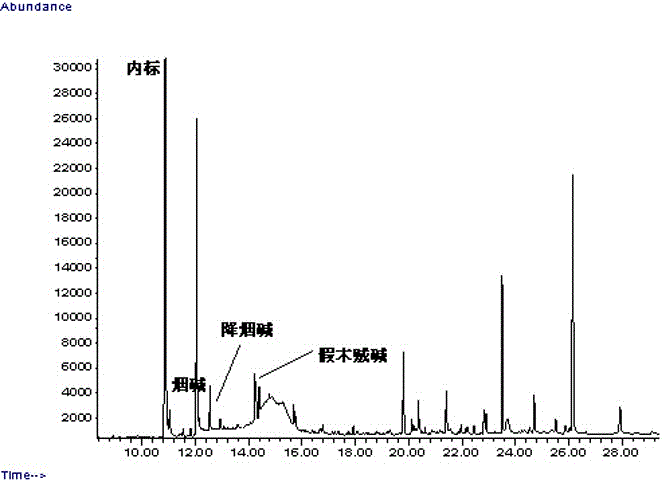
Method for determining alkaloids in tea leaves by using GC-MS (Gas Chromatography-Mass Spectrometer) method
InactiveCN105467055AOptimize detection conditionsShort detection timeComponent separationNornicotineRelative standard deviation
The invention belongs to the technical field of physiochemical detection of tea leaves and in particular relates to a method for determining alkaloids in tea leaves by using a GC-MS (Gas Chromatography-Mass Spectrometer) method. The method comprises the following step of determining nicotine and minor alkaloids (including nicotine, nornicotine, myosmine, neonicotine, neonicotine and cotinine) in tea leaf powder. The method is a method for extracting nicotine substances from the tea leaves by using a centrifugal tube filled with 0.01% triethylamine / tert-butyl methyl ether solution and analyzing the nicotine substances in the tea leaves by using a gas chromatography-mass spectrometer (GC-MS) method. When the method provided by the invention is used for detecting the content of the nicotine in the tea leaves, the method is rapid and effective, the pre-treatment is simple, an average relative standard deviation is smaller than 10 percent, and the average recycling rate of each index is 86.2 percent to 92.1 percent. The method has the advantages of rapidness and accuracy, high sensitivity and good repeatability.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Chewing gum composition for effectively eliminating nicotine accumulated in a human body
InactiveUS6958143B2Effectively eliminating nicotine accumulatedReduce productionBiocideCosmetic preparationsUrine cotinine levelChewing gum
Disclosed is a chewing gum composition capable of eliminating nicotine component from the body of a person chewing the chewing gum. Such a chewing gum composition functions to convert the nicotine produced in a human body after smoking to cotinine and to discharge the cotinine into urine. Accordingly, by chewing the chewing gum of the present invention, not only nicotine can be eliminated from the body, but also the incidence of cancer due to nicotine can be largely reduced.
Owner:TONG YANG CONFECTIONERY
Microfluidic chip for cotinine quick detection and preparation method thereof
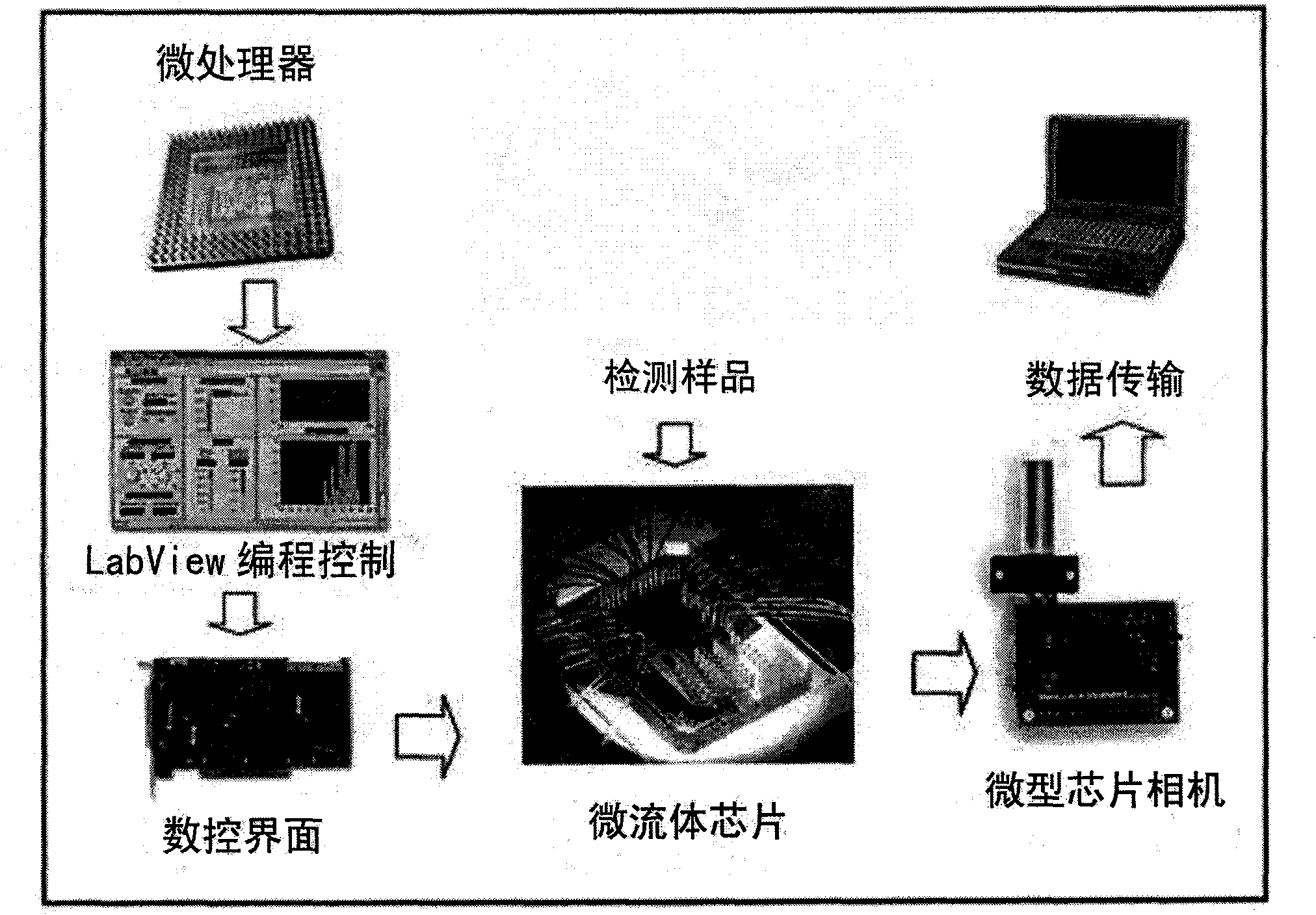
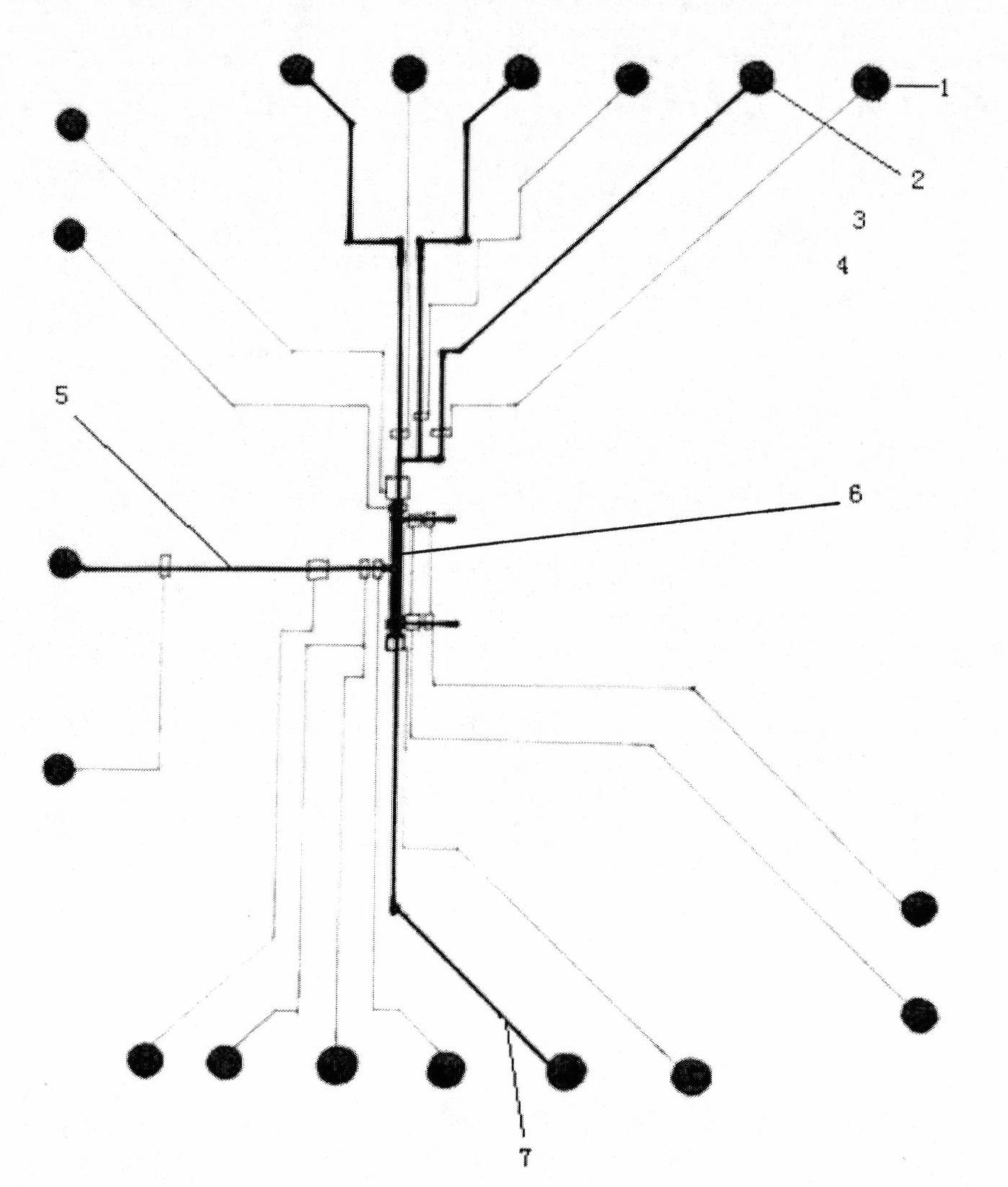
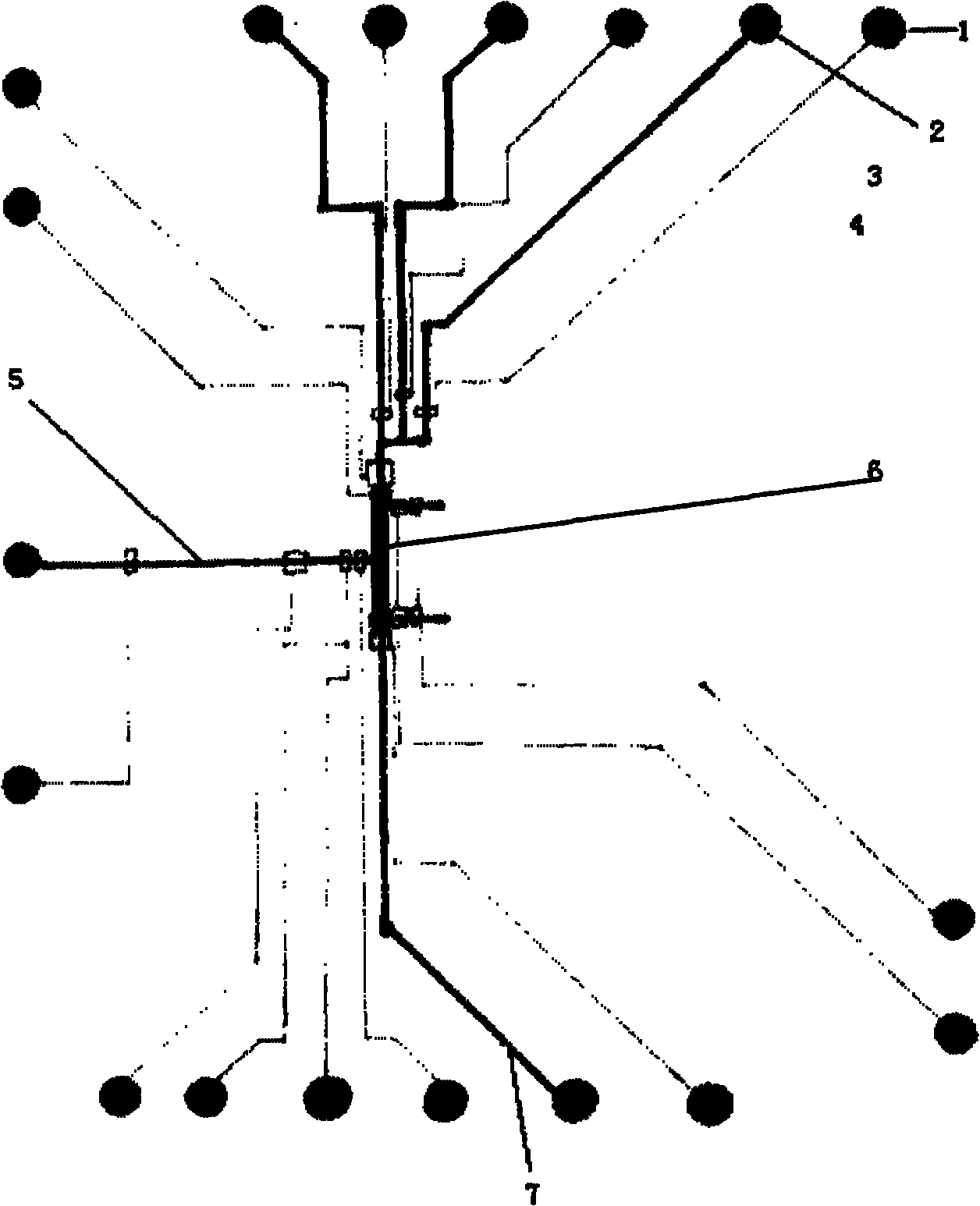
The invention belongs to the technical field of biological analysis detection, in particular to a microfluidic chip for cotinine quick detection and a preparation method thereof. In the chip, optical transparent polydimethylsiloxane and the like can be used as materials by adopting a molding method; and the chip mainly comprises a sample reaction microchannel layer, a valve control layer and a substrate layer. The chip comprises a sample enriching and immune analysis module and a signal acquisition module, wherein the sample enriching and immunity analysis module consists of a plurality of immune chromatographic column micro-analysis chambers with nanoliter volumes, and each analysis chamber fixes cotinine antibody protein or antigen to realize quick detection on the cotinine in samples from different sources. The chip has the characteristics of quickness, high efficiency and low cost, is convenient to carry, is easy to control automatically, can complete automatic signal acquisition, remote transmission and signal analysis, and is suitable for quick detection on the ingredient in the air and medical clinical detection.
Owner:FUDAN UNIV
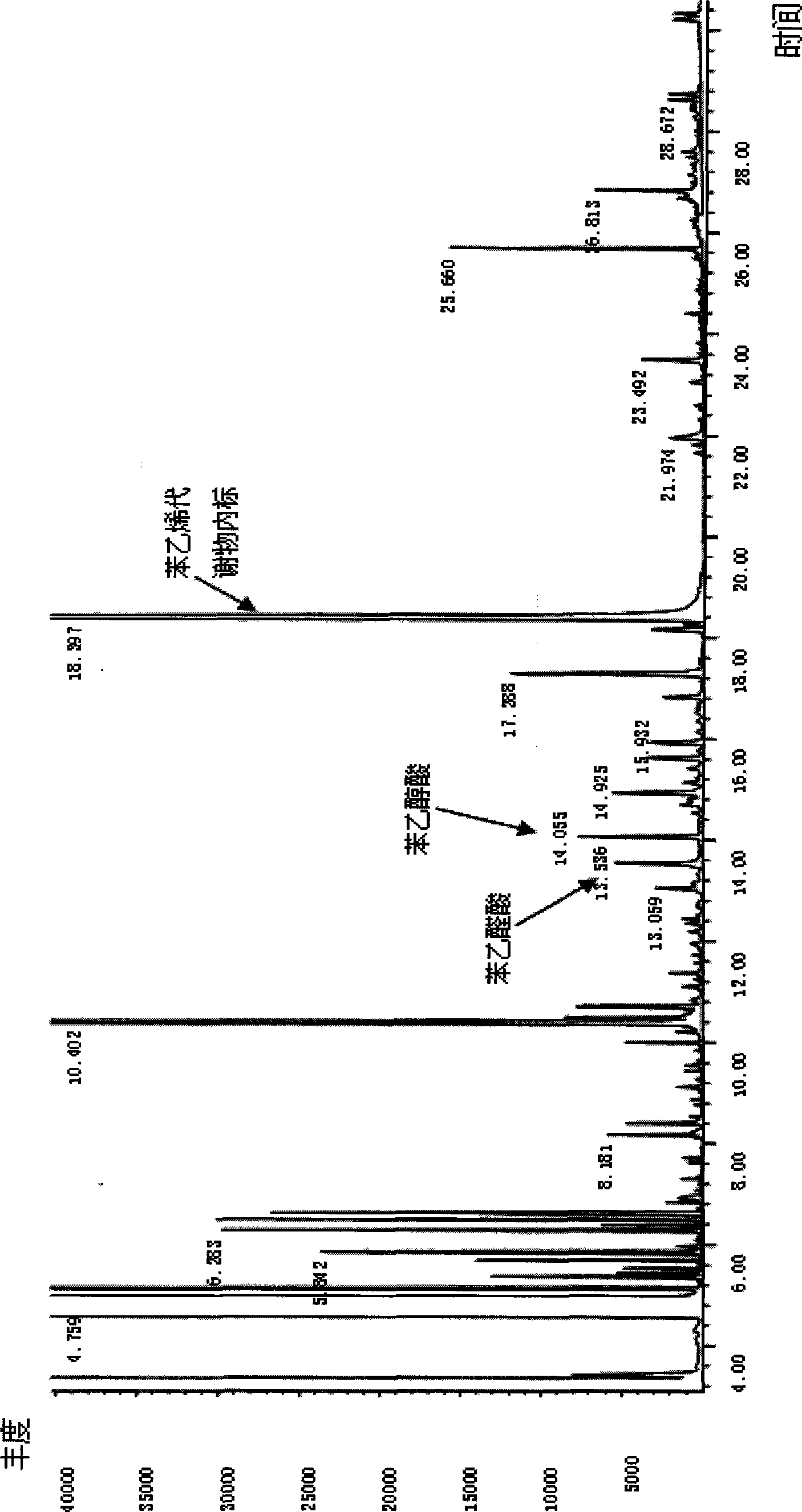
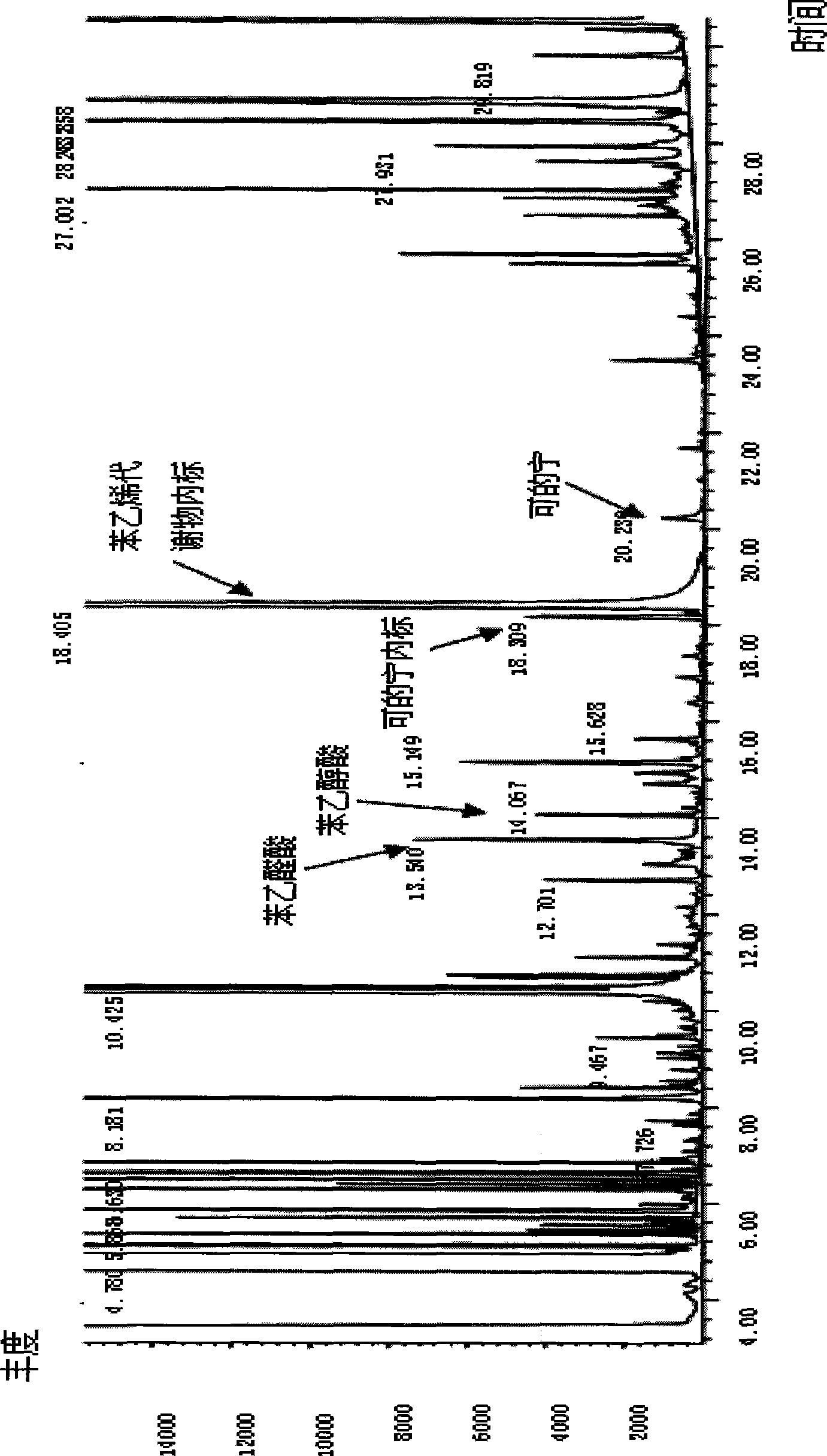
Method for simultaneously detecting cotinine, phenyl hydroxyacetic acid and phenylglyoxalic acid in human urine based on derivation method
The invention relates to the technical field of biological engineering, in particular to a method for detecting cotinine, mandelic acid and phenylglyoxalic acid in human urine simultaneously on the basis of a derivation method. The method is characterized in that the method comprises: step one, storing collected urine after centrifugal process; step two, taking the urine obtained in step one and adding the urine into internal label benzophenone, diphenylamine, hydrochloric acid and chloroform to carry out extraction through rotating centrifugation, thereby aspirating and recording organic phase as A and taking residual solution for standby; step three, taking the residual solution obtained in step two and adding the residual solution into sodium hydroxide solution and chloroform to carry out secondary extraction through rotating centrifugation, thereby aspirating and recording organic phase as B; step four, mixing the organic phase A extracted in step two and the organic phase B extracted in step three inside a glass tube, and then blowing dry both organic phases; step five, adding acetonitrile into the glass tube adopted in step four for redissolution and adding derivative reagent to carry out derivative reaction; and step six, taking the reaction solution obtained in step five to carry out gas phase chromatography-mass spectrometry combined measurement. The method has the characteristics of simplicity, convenience, quickness, reliable result, high degree of automation and convenient industrialized application.
Owner:陈枢青 +2
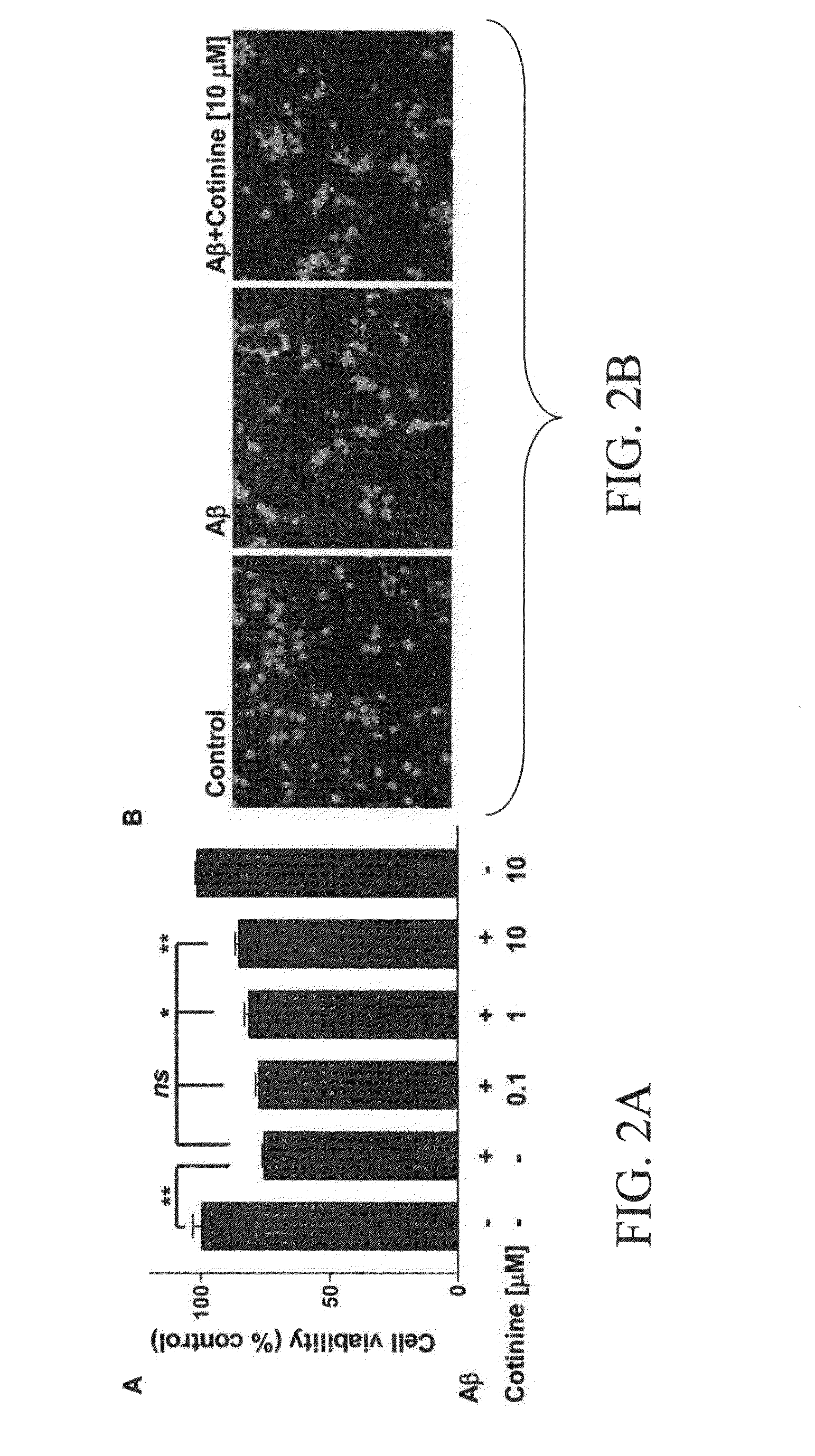
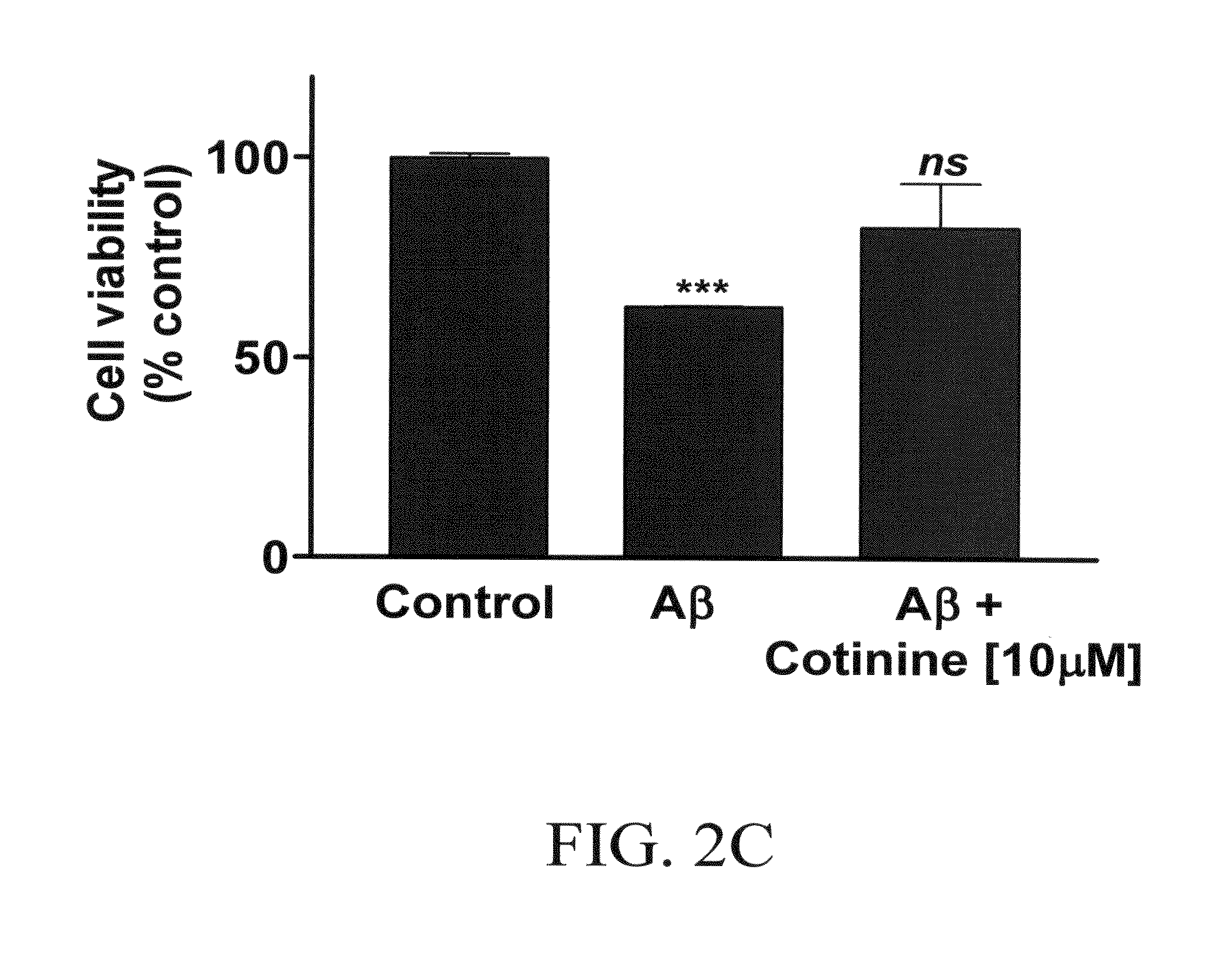
Materials and methods for diagnosis, prevention and/or treatment of stress disorders and conditions associated with abeta peptide aggregation
The subject invention concerns materials and methods for treating and / or preventing diseases associated with accumulation of Aβ peptide in neural tissue. The subject invention also concerns materials and methods for treating and / or preventing stress disorders, such as post-traumatic stress disorder (PTSD). In one embodiment, a method of the invention comprises administering a therapeutically effective amount of cotinine, or a pharmaceutically acceptable salt thereof, to a person or animal in need of treatment. The methods of the invention can be used to prevent and / or treat Alzheimer's disease, Parkinson's disease, and / or Down's syndrome. The subject invention also concerns compositions that comprise cotinine, or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier, diluent or adjuvant.The subject invention concerns materials and methods for detecting and diagnosing conditions associated with accumulation of Aβ peptide in neural tissue, such as Alzheimer's disease and Parkinson's disease, using the chemical cotinine. In one embodiment, the method comprises administering cotinine labeled with a detectable label to a person or animal. The presence of labeled cotinine in neural tissue is then determined. The level and / or location of cotinine can be analyzed and a diagnosis made. The subject invention also concerns cotinine labeled with a detectable label. In one embodiment, the cotinine is labeled with a radioisotope that can be detected by Positron Emission Tomography (PET) or single photon emission computed tomography (SPECT).
Owner:UNIV OF SOUTH FLORIDA +1
Multi-portion intra-oral dosage form with organoleptic properties
InactiveCN101843581ASolve the serious problem of high first-pass effectOrganic active ingredientsNervous disorderNornicotineActive agent
The present invention relates to a multi portion intra-oral dosage form comprising at least one pharmaceutically active agent or health promoting agent wherein at least one portion comprises a component for creating a noticeable organoleptic sensation. Of certain interest is use of sensory markers / signals as conceptual aids for a subject using the dosage form whereby the organoleptic sensation / s is / are such that it / they facilitate / s for the subject to identify a portion and differentiate between different portions thereof. Also contemplated are a dosage form, a method and a system for delivering active agents, such as nicotine and / or metabolites thereof, such as cotinine, nicotine N'-oxide, nornicotine, (S)-nicotine-N--glucuronide and mixtures, isomers, salts and complexes thereof as well as use and production of said dosage forms.
Owner:MCNEIL AB
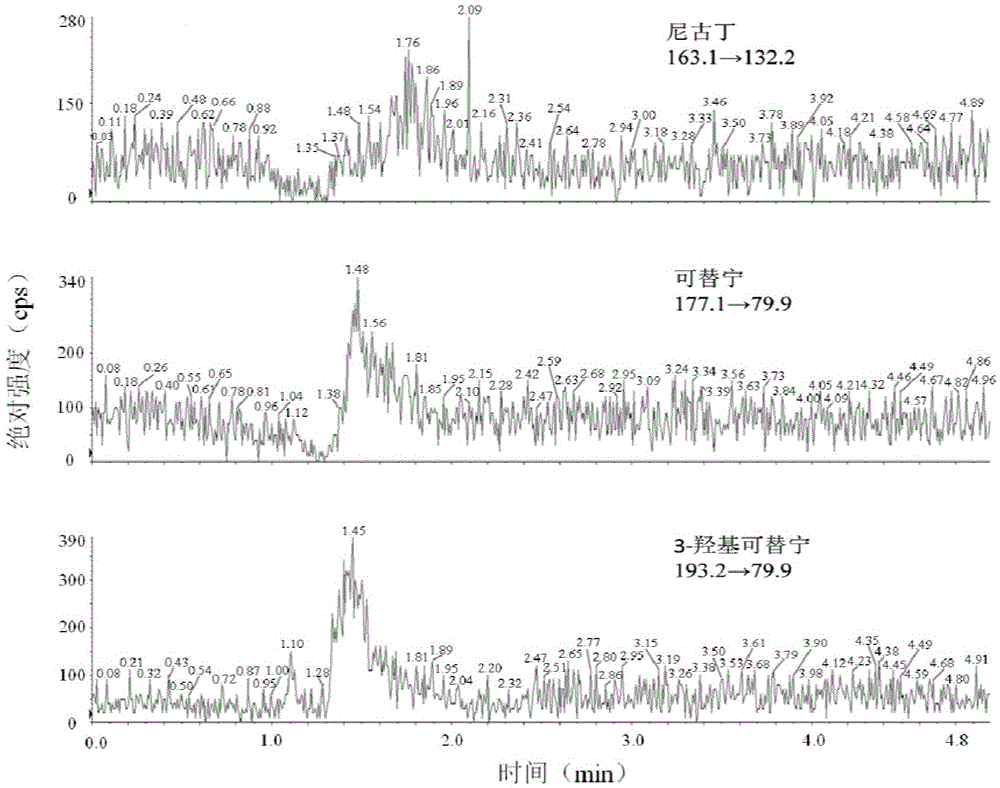
LC-MS/MS method for detecting nicotine and its metabolite in saliva
The invention discloses an LC-MS / MS method for detecting nicotine and its metabolites in saliva. The metabolites can be cotinine and 3-hydroxyl cotinine. LC-MS / MS is utilized to respectively detect a standard substance solution and a pretreated saliva sample, an isotope internal standard quantitative method is utilized to establish a calibration curve and calculate the concentration of the nicotine and its metabolites in the saliva with the concentration ratio of the standard substance solution and the internal standard substance as the X axis and the peak area ratio of the standard substance solution and the internal standard substance as the Y axis. The method is high in sensitivity, good in specificity, accurate and simple in pretreatment process and can complete separation and detection within 5 minutes, the precision and the stability meet the requirements, saliva samples are convenient to collect, no wound is caused, and the method can be used for evaluation of smoking and smoking cessation behaviors and research of environment smoke exposure degree.
Owner:SHANGHAI DIAN CLINICAL TESTING CENT
Method for measuring trace alkaloids and nitrosamines in oral tobacco products
PendingCN109975444AGuarantee the quality of smokingHigh sensitivityComponent separationIsotopeUrine cotinine level
The invention provides a method for measuring trace alkaloids and nitrosamines in oral tobacco products. The method includes the following steps that: a) oral tobacco products are sliced, and are added into to an extraction solvent and a deuterated isotope internal standard, extraction is performed with an accelerated solvent extraction method, an extract liquid is collected; b) the extract liquidand a QuECHERS reagent are subjected to vortex mixing for 1-3min, centrifuging is performed at a speed of 9000-11000 rpm for 3-7min, so that a supernate can be obtained; and c) a UPLC-HR-MS method isadopted to perform quantitative analysis on trace alkaloids and specific nitrosamines for the cigarettes in the supernate. With the method disclosed by the invention adopted, 6 trace alkaloids, namely, neonicotine, nornicotine, myosmine, cotinine, nicotelline and N-formyl nornicotine and four specific nitrosamines, namely NNN, NNK, NAT and NAB in the main stream smoke of cigarettes can be measured simultaneously. The method has the advantages of high sensitivity, high accuracy and high selectivity, and is suitable for the rapid determination of trace alkaloids and nitrosamines in batched gum-base oral tobacco products.
Owner:CHINA TOBACCO GUIZHOU IND
Methods to improve immunogenicity of antigens and specificity of antibodies
InactiveUS7147858B2Strong specificityLittle cross-reactivitySnake antigen ingredientsImmunoglobulins against animals/humansSpecific immunityUrine cotinine level
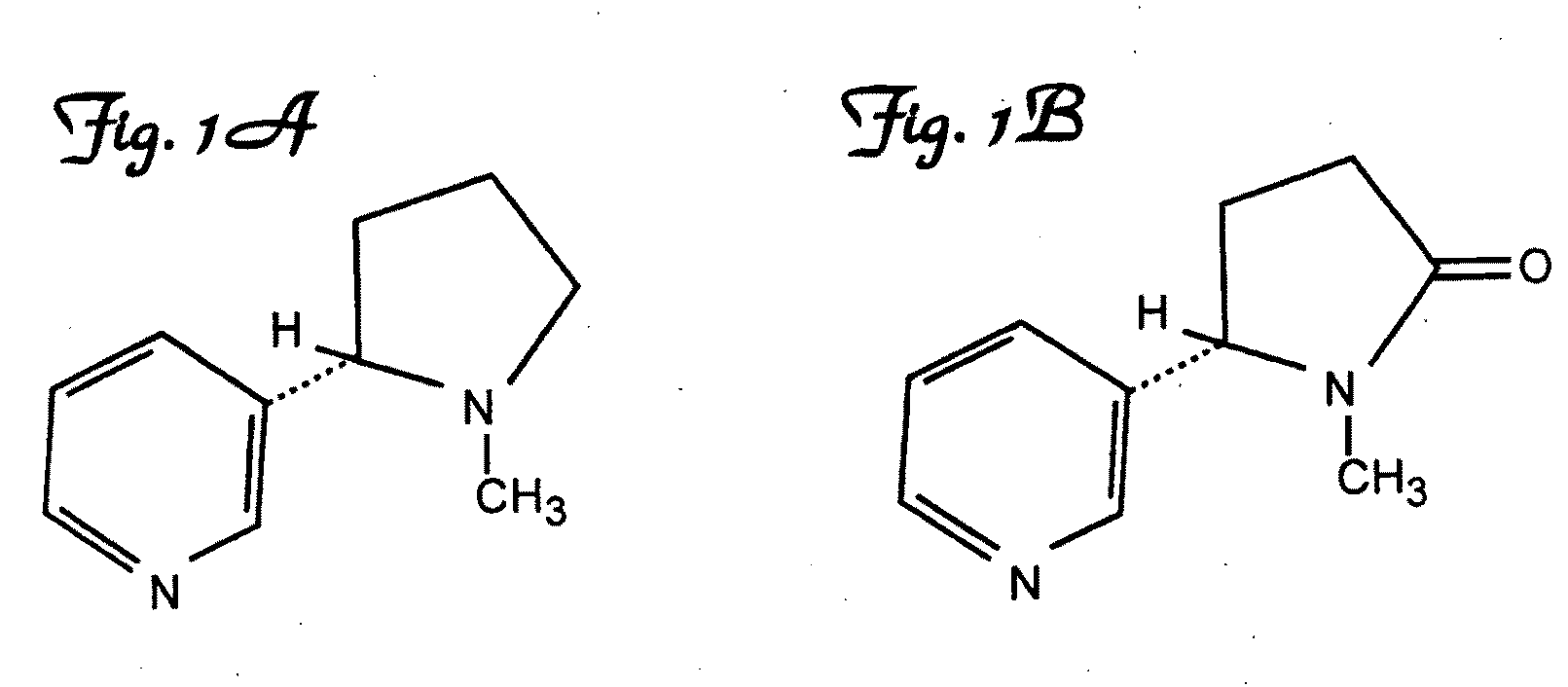
A method of improving specific immune responses to small immunogens, haptens, has been developed by changing the linkage between the hapten and carrier being used for immunization. High affinity antibodies to the hapten cotinine have been produced using this method. Antibodies to a glycated protein have also been developed, utilizing an immunogen which is composed of a glycated peptide mimic of the glycated peptide sequence which is the target epitope, wherein the peptide mimic is constructed to conformationally mimic the conformation of the peptide in the native protein, the peptide mimic contains no charged groups or other immunodominant group, and the peptide mimic is connected to a spacer sequence equivalent to a peptide spacer of between one and thirty amino acids in length, which serves to position the peptide epitope in a conformation that approximates its conformation in the native protein. In a further embodiment the peptide mimic and spacer are linked to a carrier molecule.
Owner:SEREX
Method for Detecting Second and Thirdhand Smoke
A novel method for testing if smoking has occurred within a space. Smoking is determined to have happened by testing for and confirming the presence of nicotine using a lateral flow test strip. The nicotine may be captured from secondhand smoke by exposing the condensing pad of the lateral flow tester to air within the space prior to activating the test strip. The nicotine may also be captured as part of thirdhand smoke residue on a surface within the space. A prior art commercially available lateral flow test strip used to test for the presence of cotinine within a biological sample, is used in novel ways for detecting for the past presence of nicotine smoke within a space. Improvements to the test apparatus are also disclosed.
Owner:SCHLOSSER KAREN +2
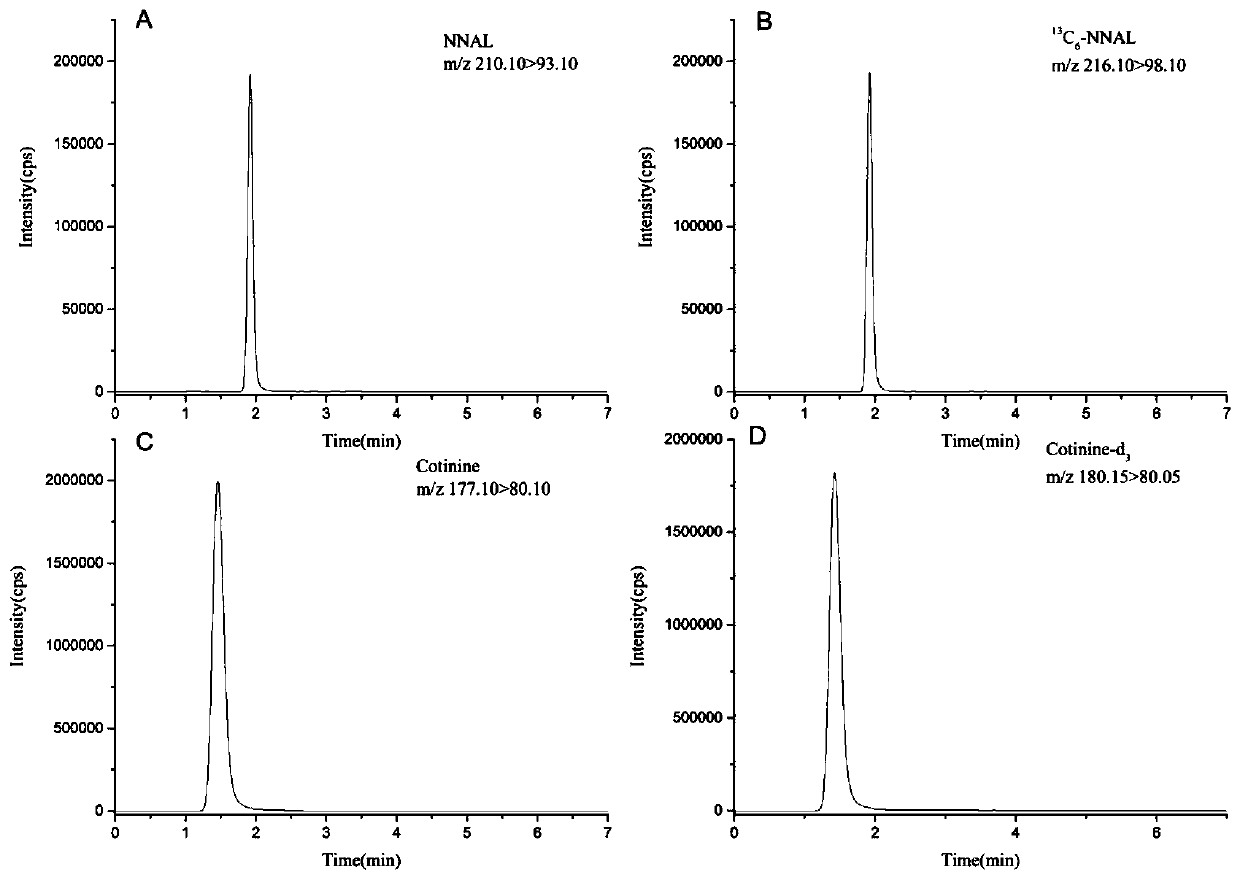
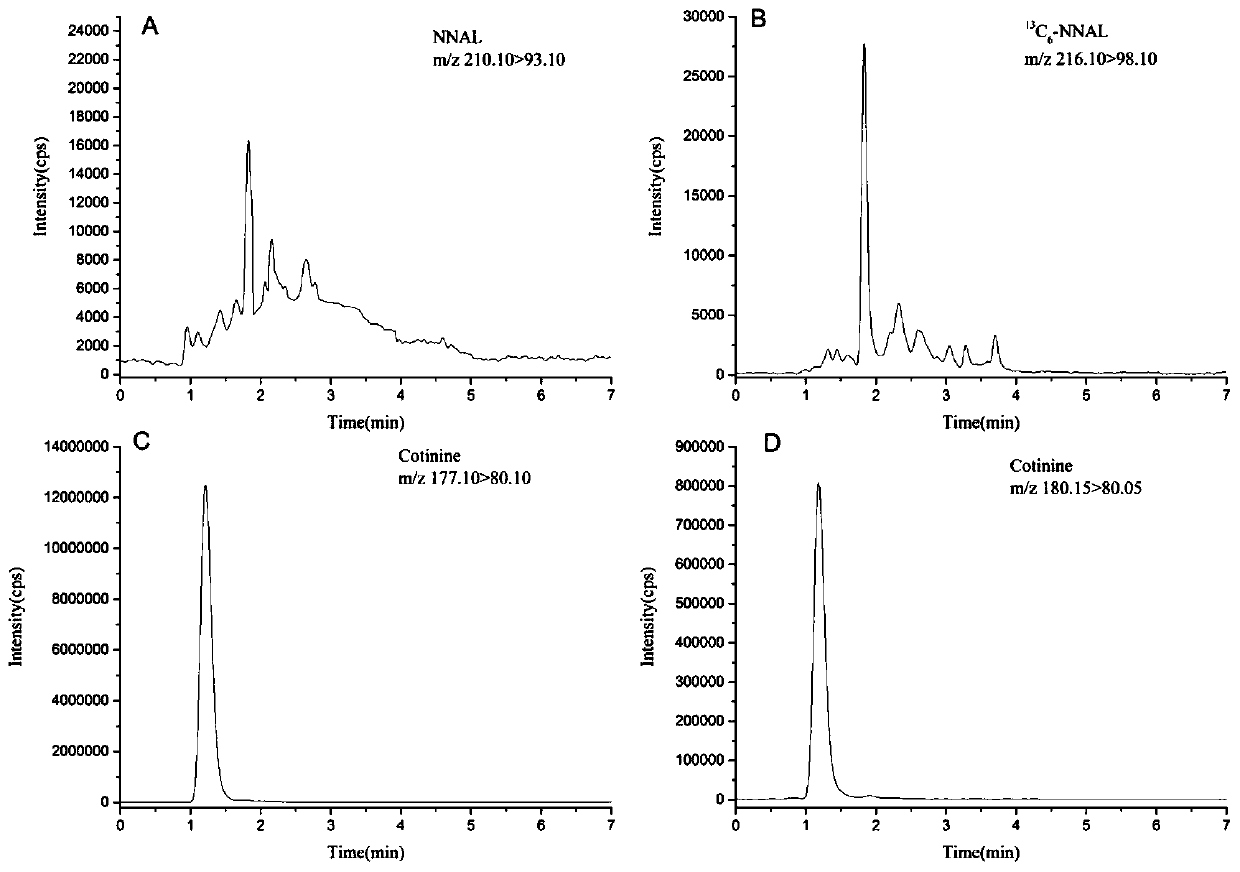
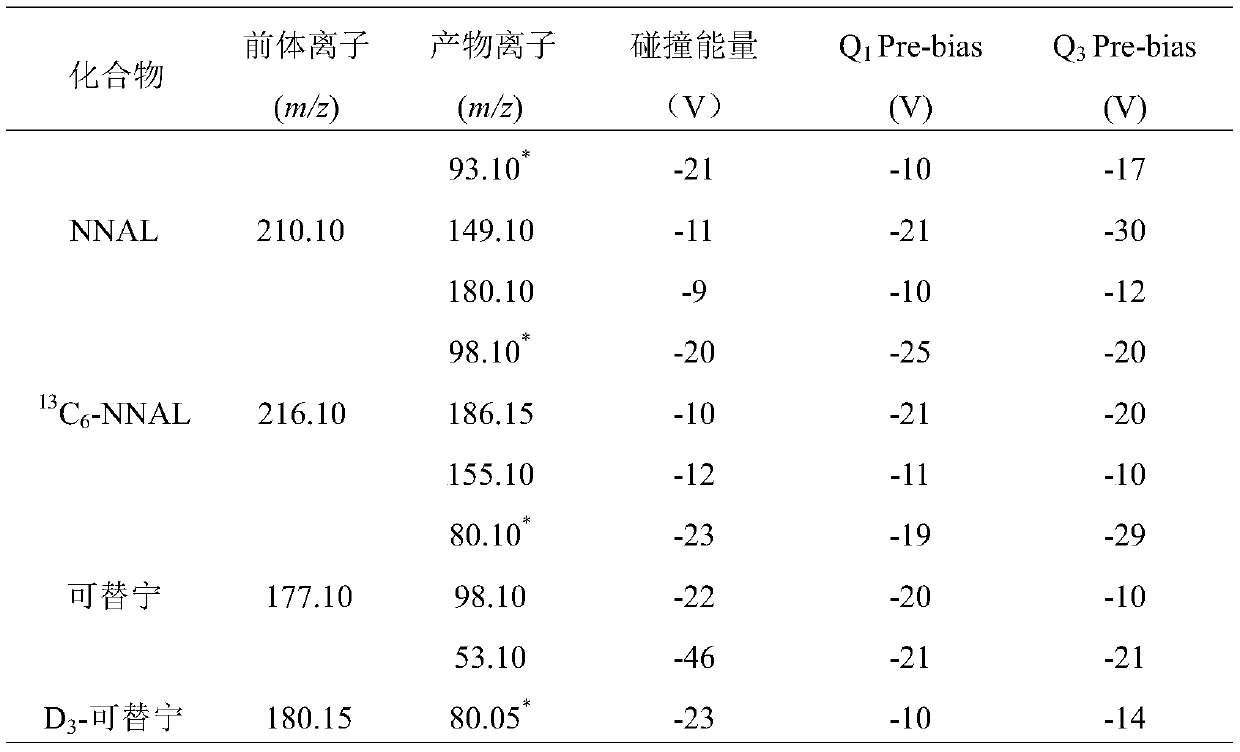
High-sensitivity and high-accuracy method for simultaneously determining NNAL and cotinine in urine
ActiveCN110333308ASolve processingTime-consuming to solveComponent separationIsotopeSolid phase extraction
The invention discloses a high-sensitivity and high-accuracy method for simultaneously determining NNAL and cotinine in urine. The method comprises the following steps: 1) preparing a single standardstock solution containing the NNAL, the cotinine, isotope-labeled NNAL and isotope-labeled cotinine; 2) preparing mixed standard sample working solutions having a series of concentrations and containing the NNAL and the cotinine, and making a standard curve; 3) taking a to-be-determined urine sample, adding a phosphate buffer solution containing an isotope-labeled internal standard, and adding 15-25 [mu]L of beta-glucuronidase for enzymolysis; and 4) taking out the sample solution subjected to the enzymolysis, naturally cooling the sample solution into the room temperature, performing solid-phase extraction, performing instrument analysis, and performing calculation according to the standard curve made in the step 2) to obtain the content of the NNAL and the cotinine. The method effectively solves the problems of complex pretreatment and long consumed time of an existing method for simultaneously testing the NNAL and the cotinine in the urine; and meanwhile, the matrix effect in the urine is greatly reduced, so that the detection sensitivity and accuracy are improved.
Owner:SICHUAN UNIV
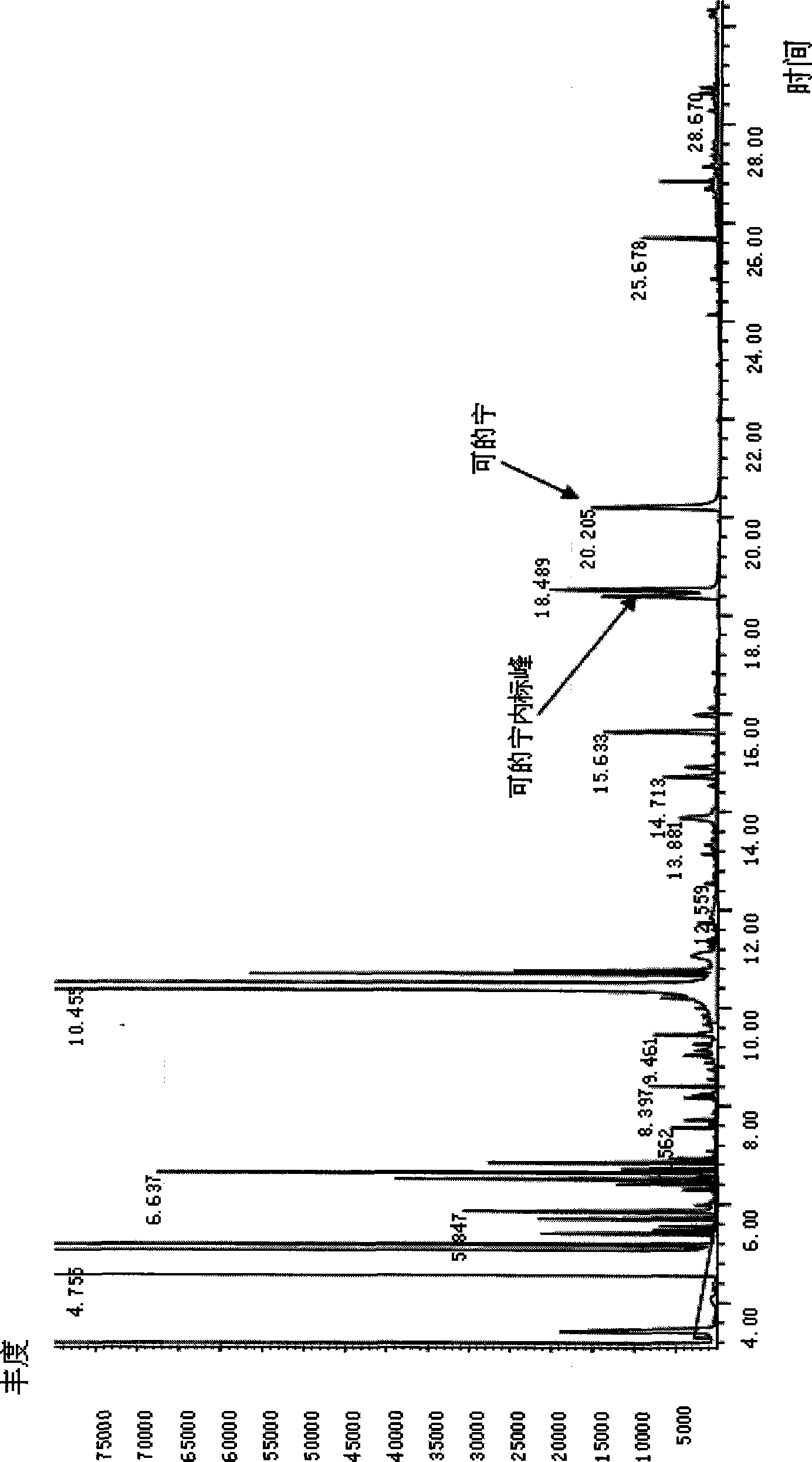
Method for determining content of cotinine in urine
ActiveCN104764826AThe pre-processing process is simpleHigh analytical sensitivityComponent separationGas phasePhase filter
The invention disclose a method for determining the content of cotinine in urine. The method comprises the following steps of: putting urine into a centrifugal tube, adding a deuterated internal standard and diluting with acetonitrile; placing the centrifugal tube in a vortex oscillation instrument, carrying out vortex oscillation and standing; sucking supernatant and filtering by an organic-phase filtering membrane, and obtaining filtrate to be determined; and preparing stock solution and working solution, and carrying out ultra-high-performance liquid chromatography-tandem mass spectrum determination. Compared with the traditional gas chromatography and mass spectrometry, the method has the beneficial effects that a mode of direct dilution with acetonitrile and vortex oscillation filtering sample injection is adopted, so that the pretreatment process is simplified and the analysis sensitivity is improved. The method has the advantages of simple operation, fastness, high accuracy and good sensitivity and repeatability.
Owner:SICHUAN BRANCH OF CHINA TOBACCO
Analysis method for rapidly detecting in-vivo metabolism marker of smoke
ActiveCN107917983AImprove enzymatic hydrolysis efficiencyChurn will notComponent separationMetaboliteSample purification
The invention relates to an analysis method for rapidly detecting an in-vivo metabolism marker of smoke. The analysis method comprises a smoke sample purification-enrichment pretreatment step and an analysis detection step. A metabolite is cotinine or 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL). The analysis method is characterized in that the metabolite is firstly hydrolyzed by virtue ofa beta-glucuronidase reactor and then is analyzed and detected by virtue of a nano-flow liquid chromatography-mass spectrometry. The innovation points of the invention are as follows: by preparing online through an enzyme reactor, the hydrolysis effect is relatively good, the experimental steps can be simplified, and the analysis speed and the analysis sensitivity can be increased; a nano-flow liquid chromatography tandem mass spectrometry is applied to the field of smoke metabolites for the first time; and the sample amount required by an experiment is extremely low, only micro-nanogram-scale samples are required under a nano-flow condition, and high-sensitivity detection and high-throughput analysis can be realized.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC +1
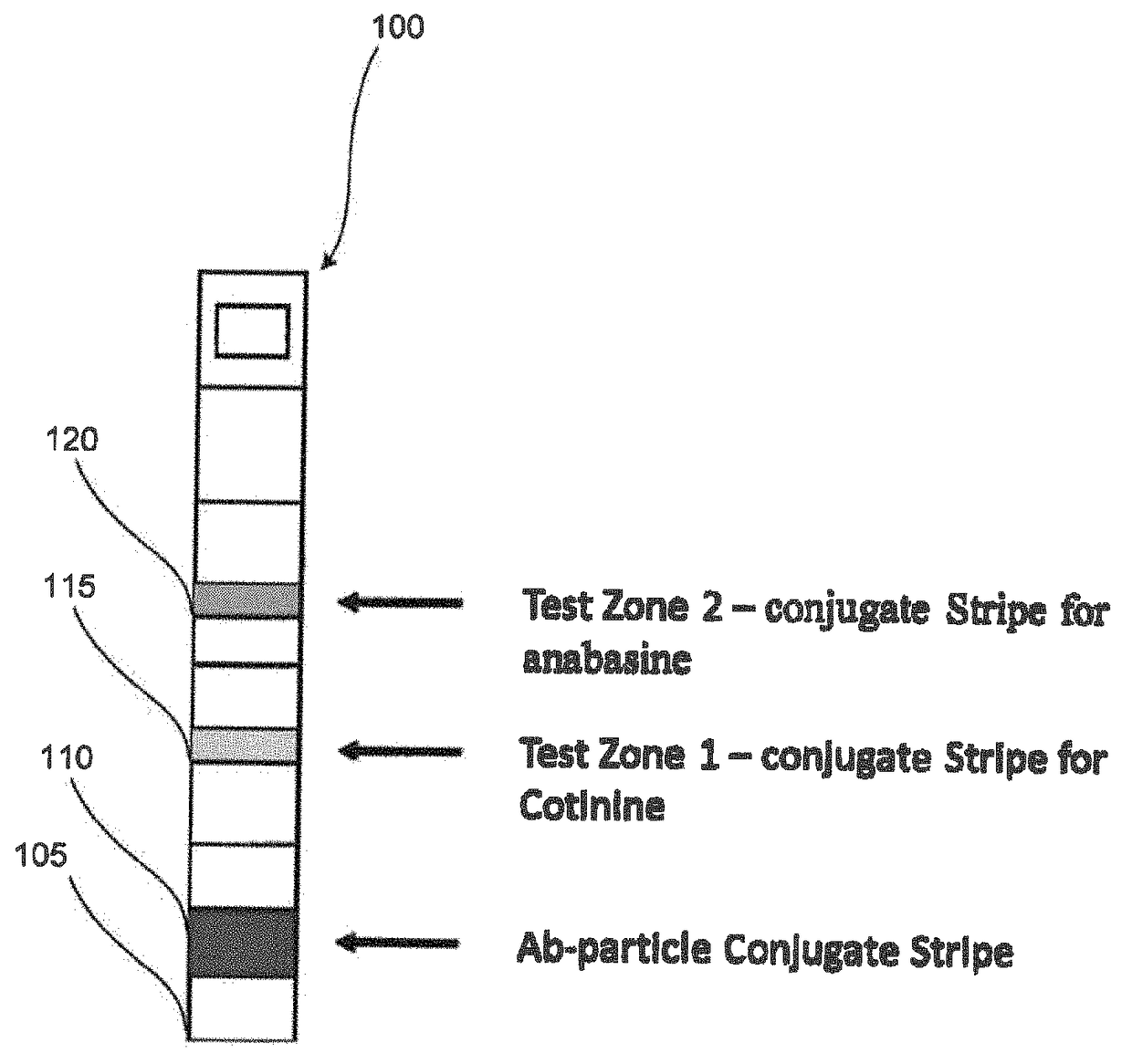
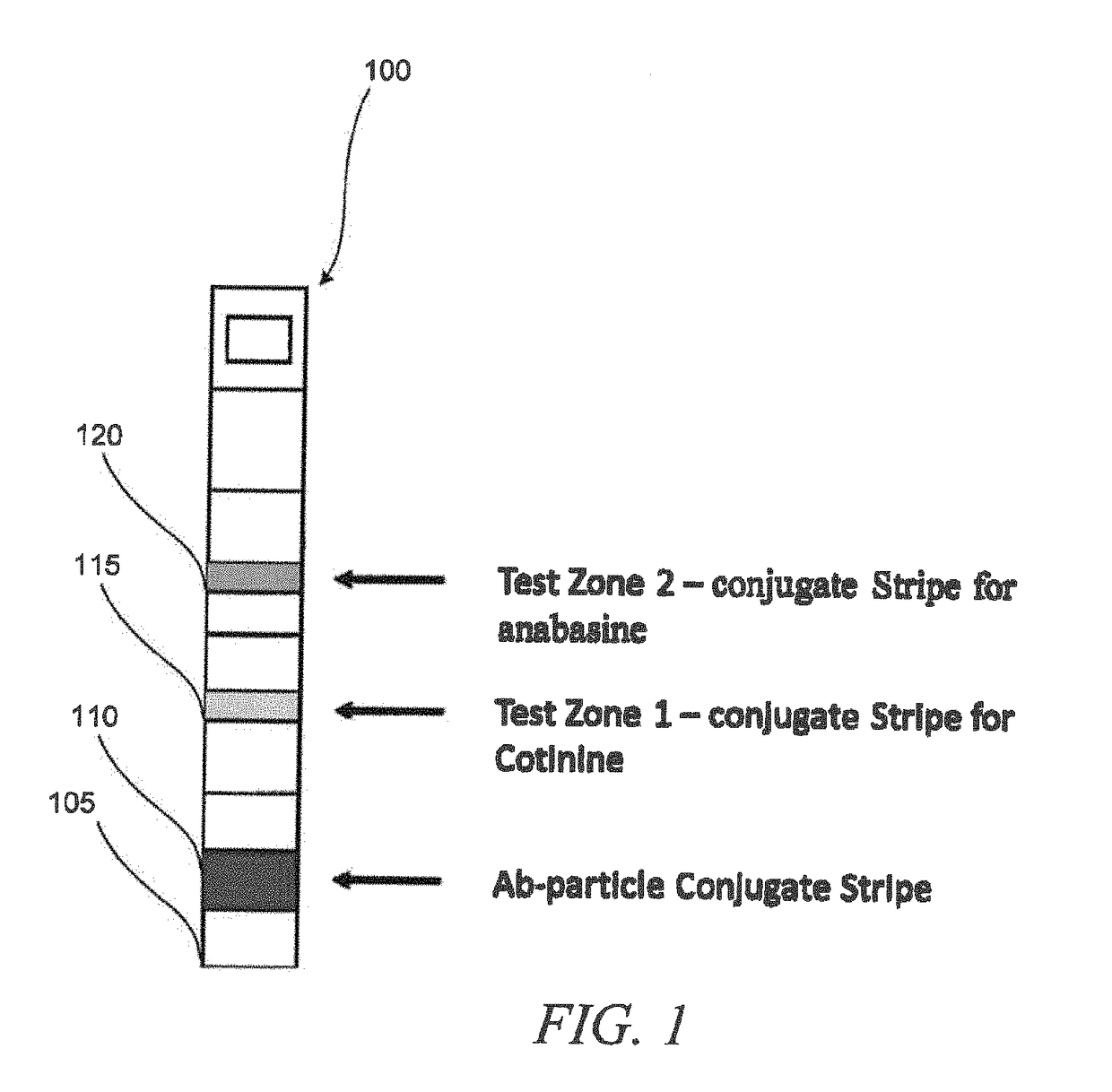
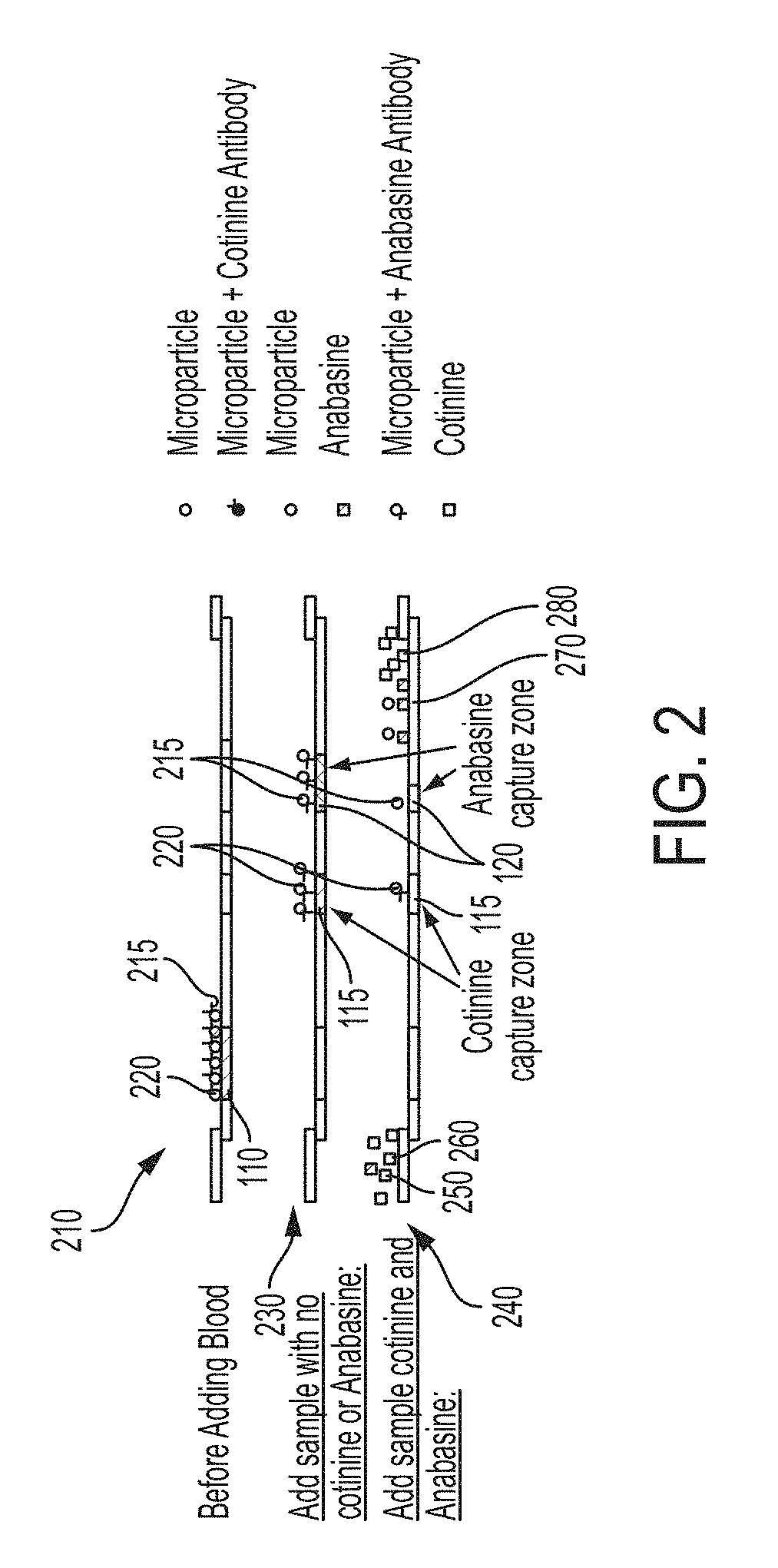
Systems and methods for distinguishing cotinine from anabasine in a point-of-care testing device
Owner:POLYMER TECH SYST INC
Method for rapidly detecting content of nicotine and cotinine in meconium of newborn
InactiveCN105548335AThe pre-processing process is simpleSimplify preprocessing stepsMaterial analysis by electric/magnetic meansChromatographic separationMeconium sample
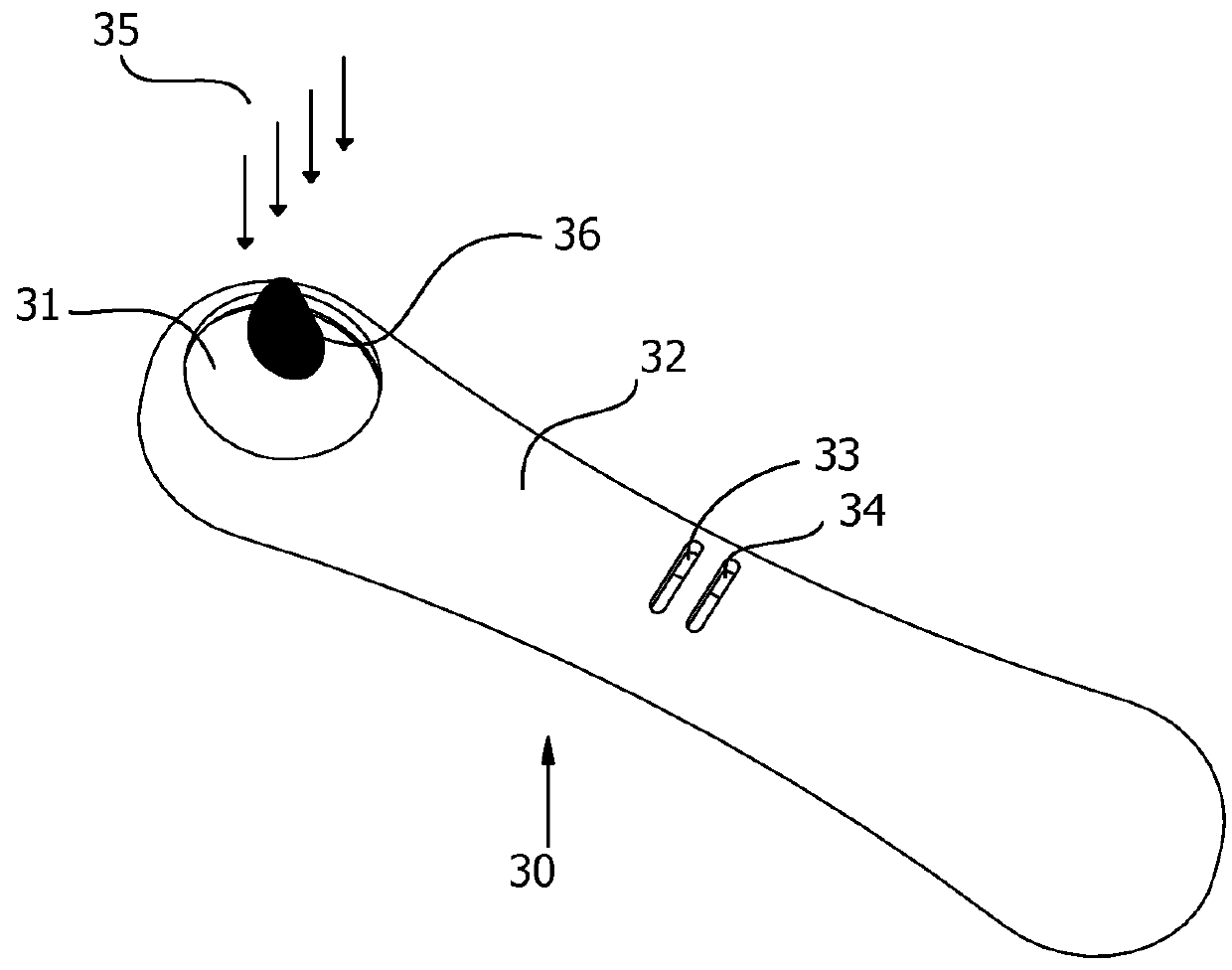
The invention discloses a method for rapidly detecting the content of nicotine and cotinine in meconium of a newborn. The method comprises the steps: extracting a meconium sample of the newborn by using a medical cotton swab; dropwise adding an isotopic internal standard methanol solution containing the nicotine and cotinine to a part, away from the sample by 0.5mm to 1.5mm, on the medical cotton swab as an extracting ionization reagent, so as to ionize the sample and generate atomization, and enabling a mass-spectrum centerline to be parallel to a toothpick at the distance of 0.5cm to 5cm; starting up a mass spectroscopy system, and setting as a cation mode. The method disclosed by the invention has the advantages that a pretreatment process for the sample is simplified, sample pretreatment such as extraction and chromatographic separation is not carried out, and the newborn is subjected to direct ionization mass spectroscopy through swabbing an electrospray mass spectrum by adopting the medical cotton swab, so that the whole analysis process is greatly simplified; the medical cotton swab is clean and hygienic and is particularly applicable to clinical large-batch analysis. Sample pretreatment steps are simplified, and direct ionization is carried out, so that the correctness and accuracy of assaying are greatly improved.
Owner:江西省妇幼保健院
LC-MS/MS kit for detecting nicotine and its metabolites in saliva
The invention discloses an LC-MS / MS kit for detecting nicotine and its metabolites in saliva. The metabolites are cotinine and 3-hydroxyl cotinine. The kit includes the following reagents: two types of eluent, mixed standard liquid A, internal standard liquid B, two types of diluent, an organic solvent and quality control substance, wherein the eluent A is 0.1% v / v of a formic acid aqueous solution, the eluent B is 0.1% v / v of a formic acid methanol solution, the mixed standard liquid A is a methanol solution containing nicotine, cotinine and 3-hydroxyl cotinine, the internal standard liquid B is an internal standard solution containing deuterated cotinine, the dilute 1 is non-smoker's saliva, the diluent 2 is methanol, the organic solvent is acetonitrile, and the quality control substance is non-smoker's saliva containing nicotine, cotinine and 3-hydroxyl cotinine. The kit is high in detection sensitivity, good in specificity, accurate and simple in pretreatment process, saliva samples are convenient to collect, no wound is caused, and the kit can be used for evaluation of smoking and smoking cessation behaviors and research of environment smoke exposure degree.
Owner:SHANGHAI DIAN CLINICAL TESTING CENT
Novel bacterial strain for nicotine degradation-Pseudomonas ZUTSKD and uses thereof
InactiveCN100537747CIncrease concentrationBacteriaMicroorganism based processesMicrobiologyPseudomonas
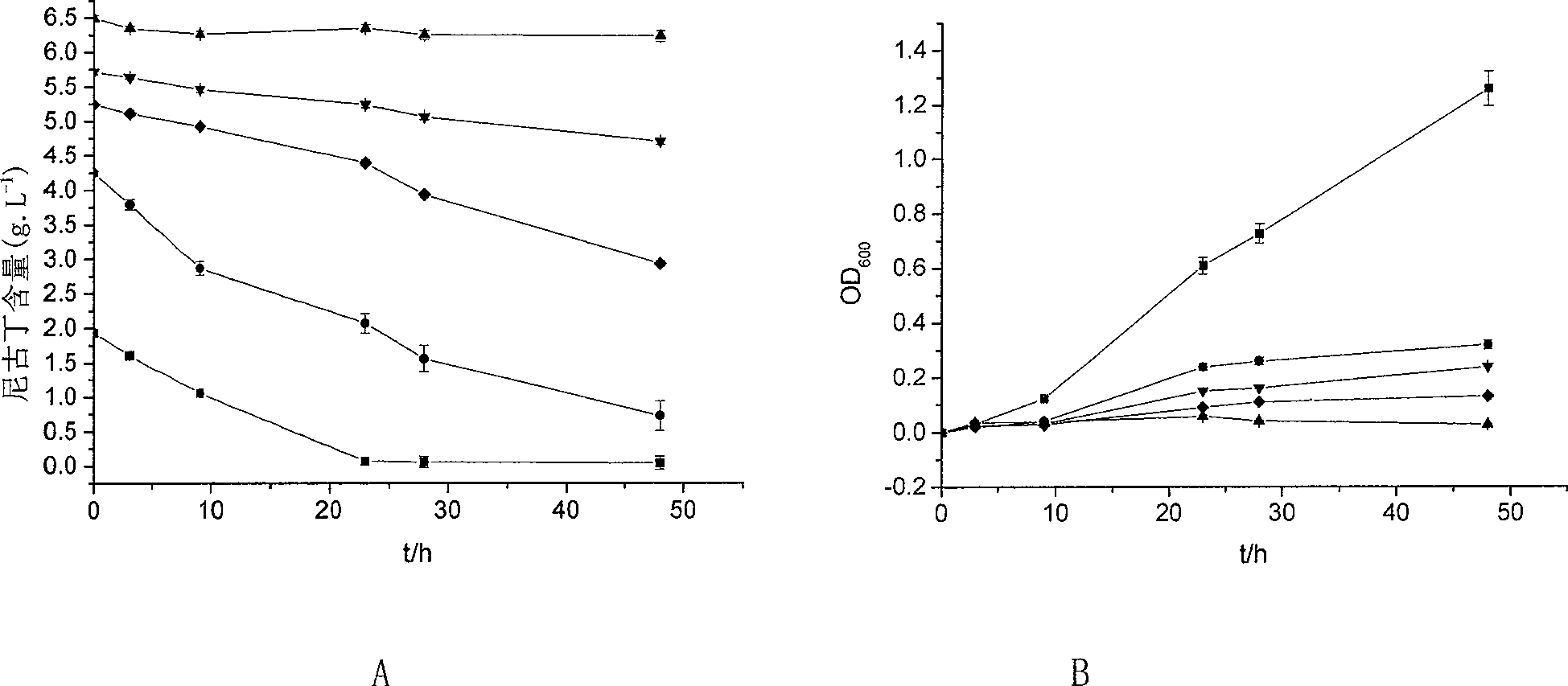
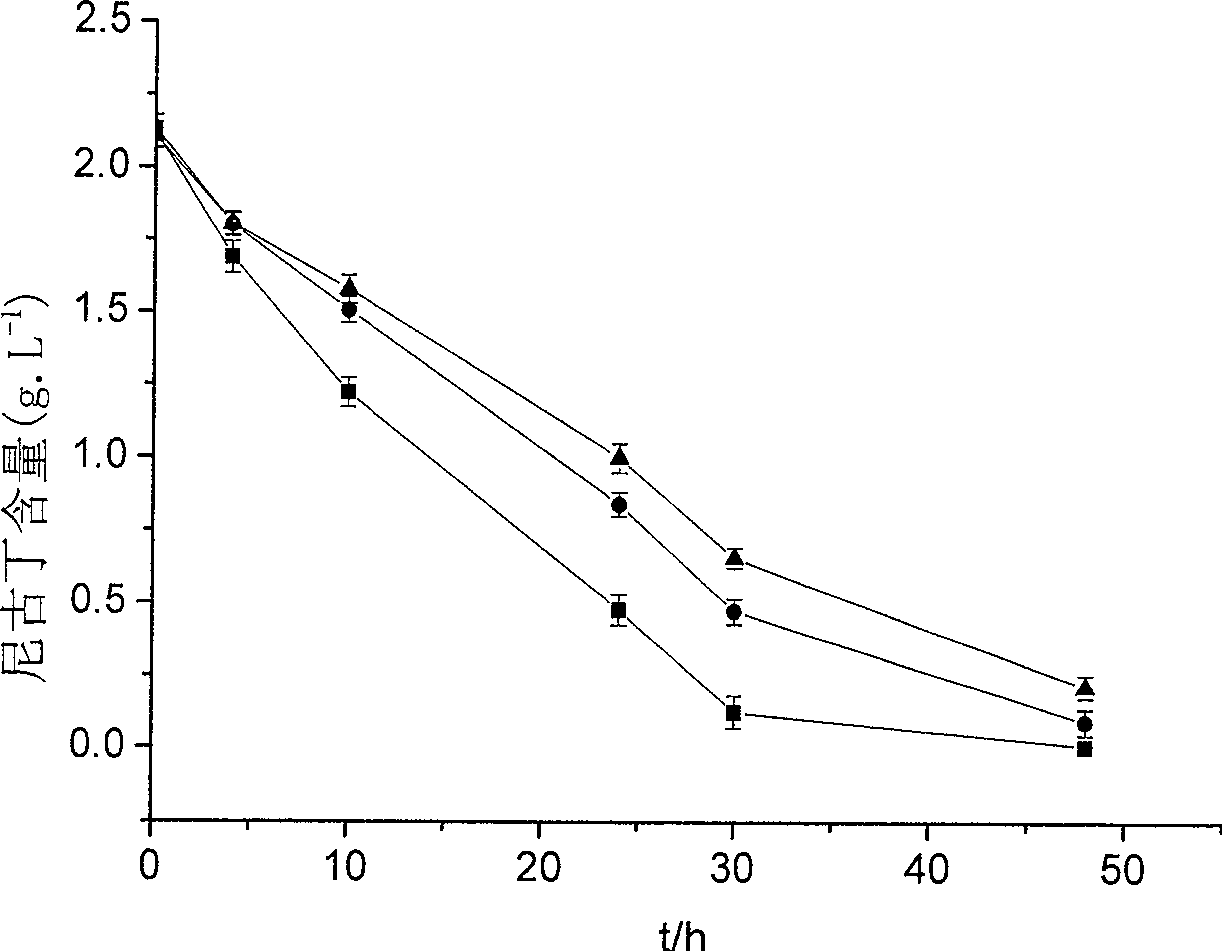
The invention provides a new strain of nicotine degrading bacteria—Pseudomonas ZUTSKD and its application. Pseudomonas sp. ZUTSKD (Pseudomonas sp. ZUTSKD), preserved in China Center for Type Culture Collection, date of preservation is June 18, 2007, preservation number CCTCCNO: M 207083. The bacteria can degrade nicotine to produce intermediate products such as 2,3'-Bipyridine, cotinine, 3-(3,4-dihydro-2H-pyrrol-5-yl)-Pyridine and 3-Pyridinecarboxylic acid, and can tolerate the highest nicotine With a concentration of 5.5g / L, it can degrade nicotine in waste tobacco powder, and has a certain application prospect in industry.
Owner:ZHEJIANG UNIV OF TECH
Method for measuring cotinine in body fluid through molecular-imprinting solid-phase extraction-liquid chromatography
InactiveCN101963600AHigh selectivityHigh sensitivityComponent separationSorbentSolid phase extraction
The invention relates to a method for measuring the cotinine in a body fluid, in particular to a method for measuring the cotinine in the body fluid through molecular-imprinting solid-phase extraction-liquid chromatography. The method sequentially comprises the following steps of: (1) synthesizing a cotinine molecular-imprinting polymer by using the cotinine as a template; (2) preparing a solid-phase extraction small column by using the synthesized polymer as a solid-phase extraction adsorbent; (3) sequentially leaching the solid-phase extraction small column by adopting an acetonitrile solution containing organic acid and water, alcohol and a buffer solution; (4) concentrating an eluant to dryness, and adding an acetate buffer solution to obtain a detected liquid; and (5) measuring the cotinine in the detected liquid by utilizing high-efficiency liquid chromatography. In the invention, the cotinine in the body fluid is firstly completely extracted and then eluted through solid-phase extraction, thereby realizing the rapid and accurate quantitative analysis of the cotinine thereof.
Owner:CHINA TOBACCO JIANGXI IND CO LTD
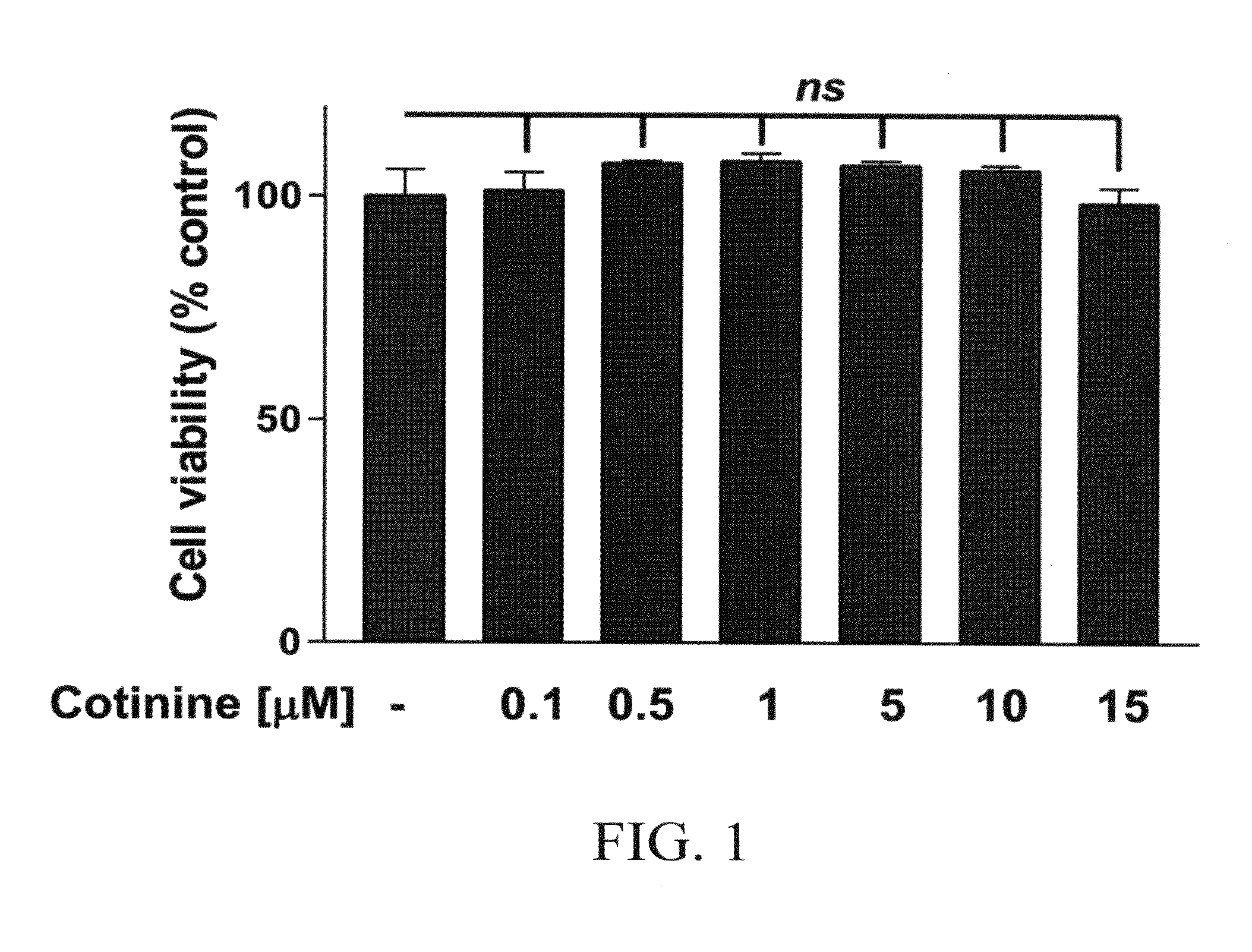
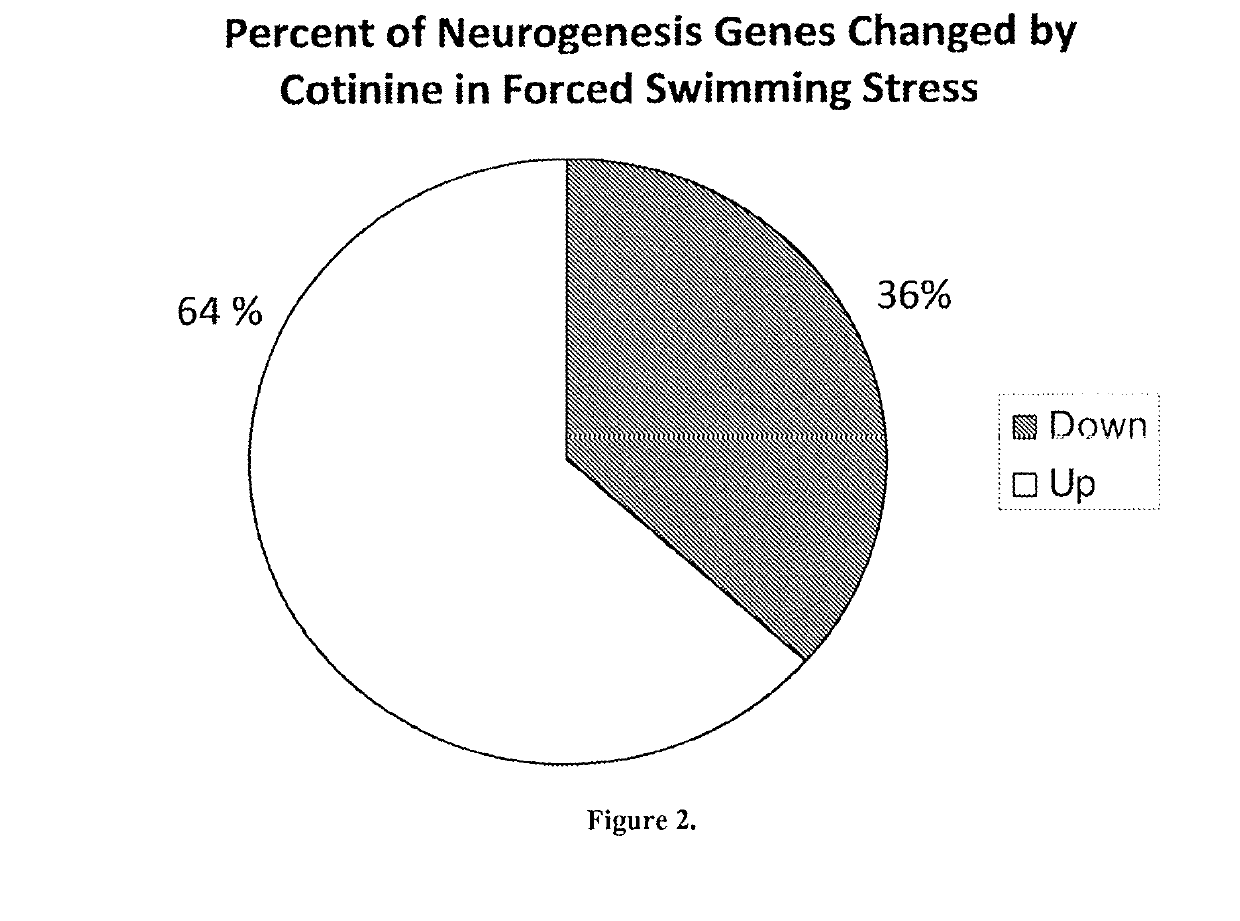
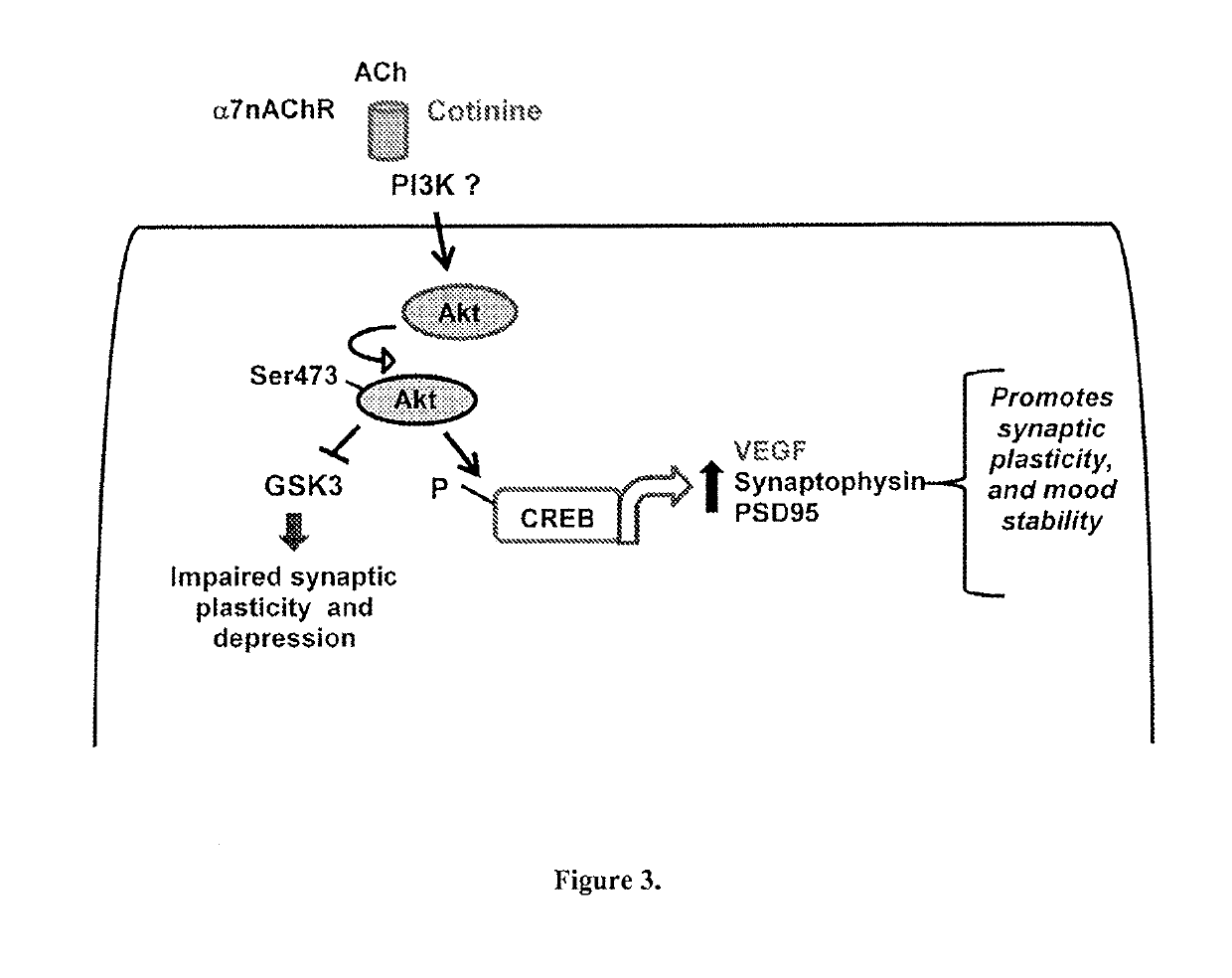
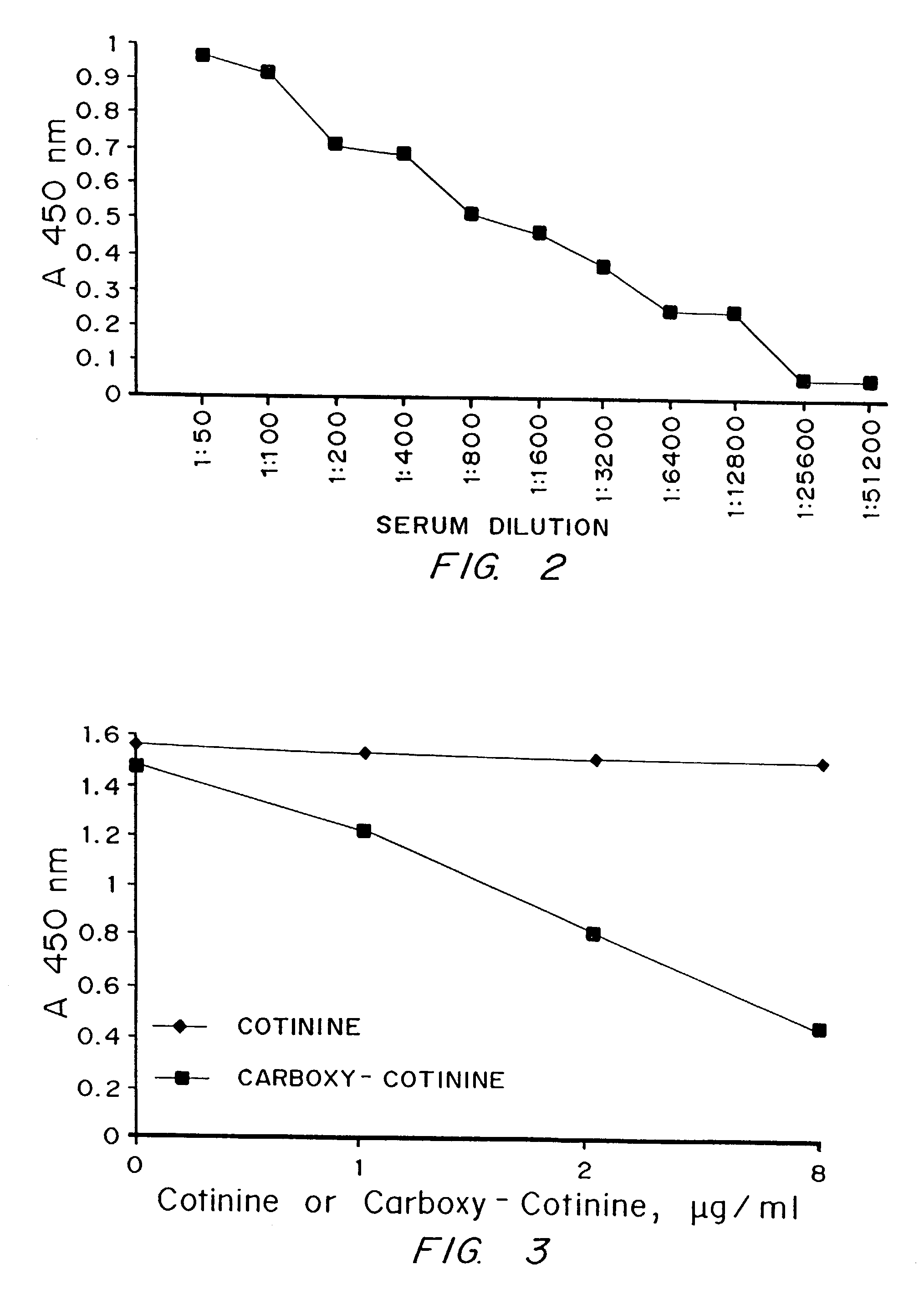
Use of cotinine in treating or preventing neurogenesis deficits and enhancing neurogenesis
ActiveUS10307411B2Reduce expressionReduce negative impactNervous disorderHeterocyclic compound active ingredientsNeurogenesisChemotherapy induced
A method of inhibiting or treating chemotherapy-induced cognitive dysfunction comprising administering a therapeutically effective amount of cotinine to a cancer patient experiencing chemotherapy-induced cognitive dysfunction.
Owner:UNIV OF SOUTH FLORIDA +1
Methods to improve immunogenicity of antigens and specificity of antibodies
InactiveUS7303750B2Strong specificityLittle cross-reactivityImmunoglobulins against animals/humansSnake antigen ingredientsSpecific immunityUrine cotinine level
A method of improving specific immune response to small immunogens, haptens, has been developed by changing the linkage between the hapten and carrier being used for immunization. High affinity antibodies to cotinine have been produced using this method. Antibodies to a glycated protein have also been developed, utilizing an immunogen which is composed of a glycated peptide mimic of the glycated peptide sequence which is the target epitope, wherein the peptide mimic is constructed to conformationally mimic the conformation of the peptide in the native protein, the peptide mimic contains no charged groups or other immunodominant group, and is connected to a spacer sequence equivalent to a peptide spacer of between one and thirty amino acids in length, which serves to position the peptide epitope in a conformation that approximates its conformation in the native protein. In another embodiment, the peptide mimic and spacer are linked to a carrier molecule.
Owner:SEREX
Chewing gum composition for eliminating nicotine
InactiveUS20030129145A1Soften physical propertyImprove stabilityBiocideOrganic active ingredientsUrine cotinine levelChewing gum
Disclosed is a chewing gum composition capable of eliminating nicotine component from the body of a person chewing the chewing gum. Such a chewing gum composition functions to convert the nicotine produced in a human body after smoking to cotinine and to discharge the cotinine into urine. Accordingly, by chewing the chewing gum of the present invention, not only nicotine can be eliminated from the body, but also the incidence of cancer due to nicotine can be largely reduced.
Owner:TONG YANG CONFECTIONERY
Ointment-form snuff and preparation method thereof
InactiveCN106617263AIncrease the effective surface areaPromote absorptionOrganic active ingredientsNervous disorderParaffin waxWhite petrolatum
The invention belongs to the field of tobacco products, and discloses ointment-form snuff and a preparation method thereof. The ointment-form snuff is prepared from the following materials in percentage by mass: 43-44% of paraffin wax, 17-21% of petroleum jelly, 2.4-3% of penetration enhancer, 6.2-7.8% of nicotine, 1.9-2.5% of cotinine, 2.3-5.1% of neophytadiene, 0.34-0.49% of furfural, 0.5-0.9% of ionol, 0.43-0.56% of megastigmatrienone and the like; and the pH value of the ointment-form snuff is 4.7-5.9. The ointment-form snuff provided by the invention, as a substitute of conventional cigarettes, is similar with the conventional cigarettes in such qualities as aroma, strength, taste and the like. The ointment-form snuff is relatively small in size, convenient to carry and broad in application scope, and the ointment-form snuff, which does not need to be burned, can avoid fire disaster, avoid influence of smoke to people around and avoid secondhand smoking.
Owner:GUANGDONG BRANCH OF CHINA TOBACCO GENERAL
Therapeutic cotinine compositions
InactiveUS20100143270A1Increase productionEffective therapeutic interventionBiocideAntipyreticUrine cotinine levelPharmacology
The present invention provides methods and compositions for treating acute and / or chronic inflammation with cotinine or a pharmaceutically acceptable salt thereof.
Owner:UNIV OF LOUISVILLE RES FOUND INC
A method for measuring cotinine content in urine
ActiveCN104764826BThe pre-processing process is simpleHigh analytical sensitivityComponent separationPhase filterUrine cotinine level
The invention discloses a method for determining the content of cotinine in urine, comprising the following steps: pipetting the urine into a centrifuge tube, adding a deuterated internal standard, and diluting with acetonitrile; placing the centrifuge tube in a vortex shaker, vortexing Shake and let stand; draw the supernatant and filter it through an organic phase filter membrane, and the filtrate is to be tested; prepare the stock solution and working solution, and use ultra-high performance liquid chromatography-tandem mass spectrometry for determination. Compared with the traditional gas chromatography-mass spectrometry method, the present invention adopts the method of direct dilution with acetonitrile and vortex vibration filtering for sample injection, which simplifies the pretreatment process and improves the analytical sensitivity. The method has the advantages of simple operation, rapidity, accuracy, good sensitivity and repeatability.
Owner:SICHUAN BRANCH OF CHINA TOBACCO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com