Method for simultaneously detecting cotinine, phenyl hydroxyacetic acid and phenylglyoxalic acid in human urine based on derivation method
A technology for detecting human body and phenylglycolic acid, applied in the field of bioengineering, can solve the problems of high detection cost, difficult contaminant contact degree, different types, etc., and achieve the effects of high detection sensitivity, convenience and easy availability, and low detection cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
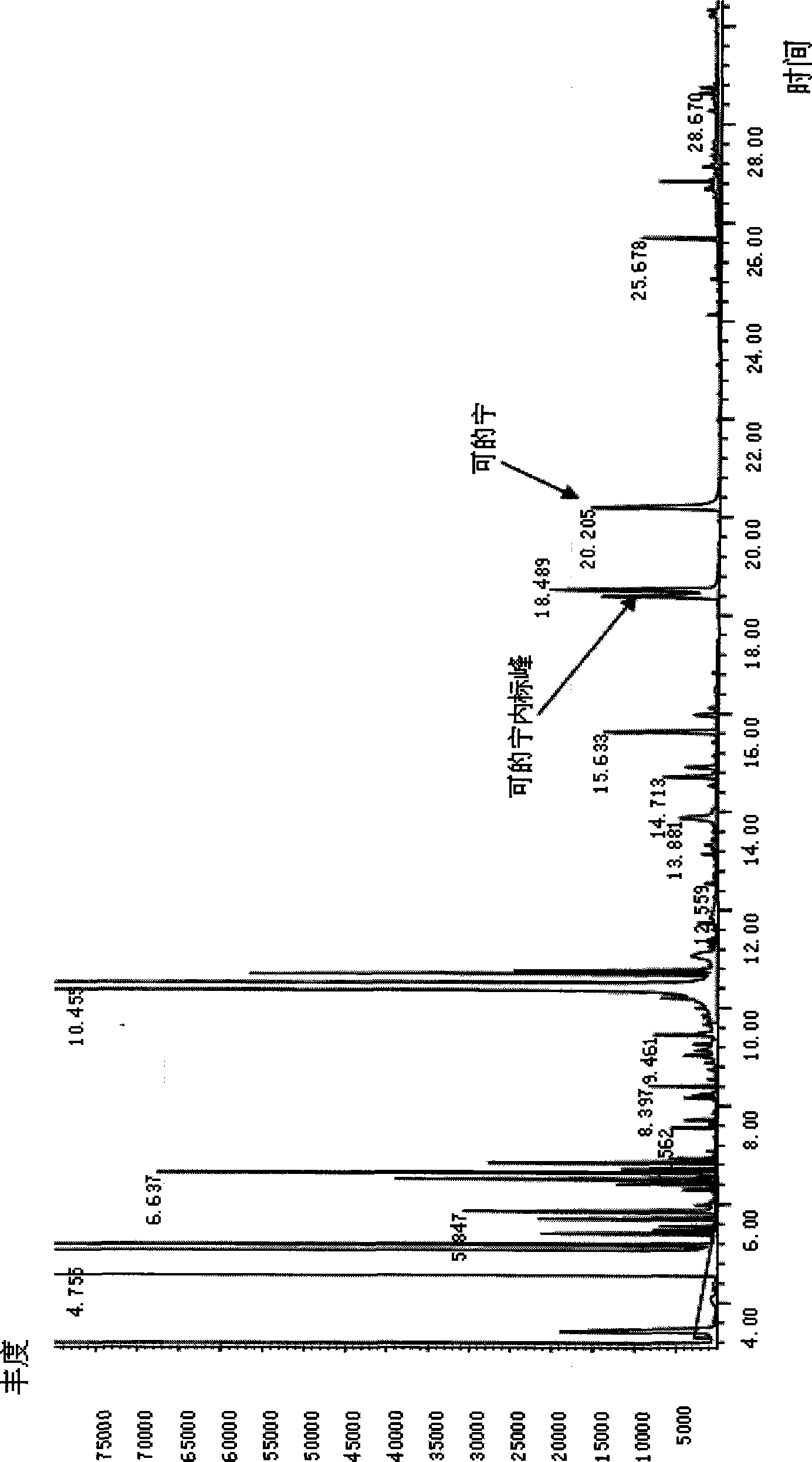
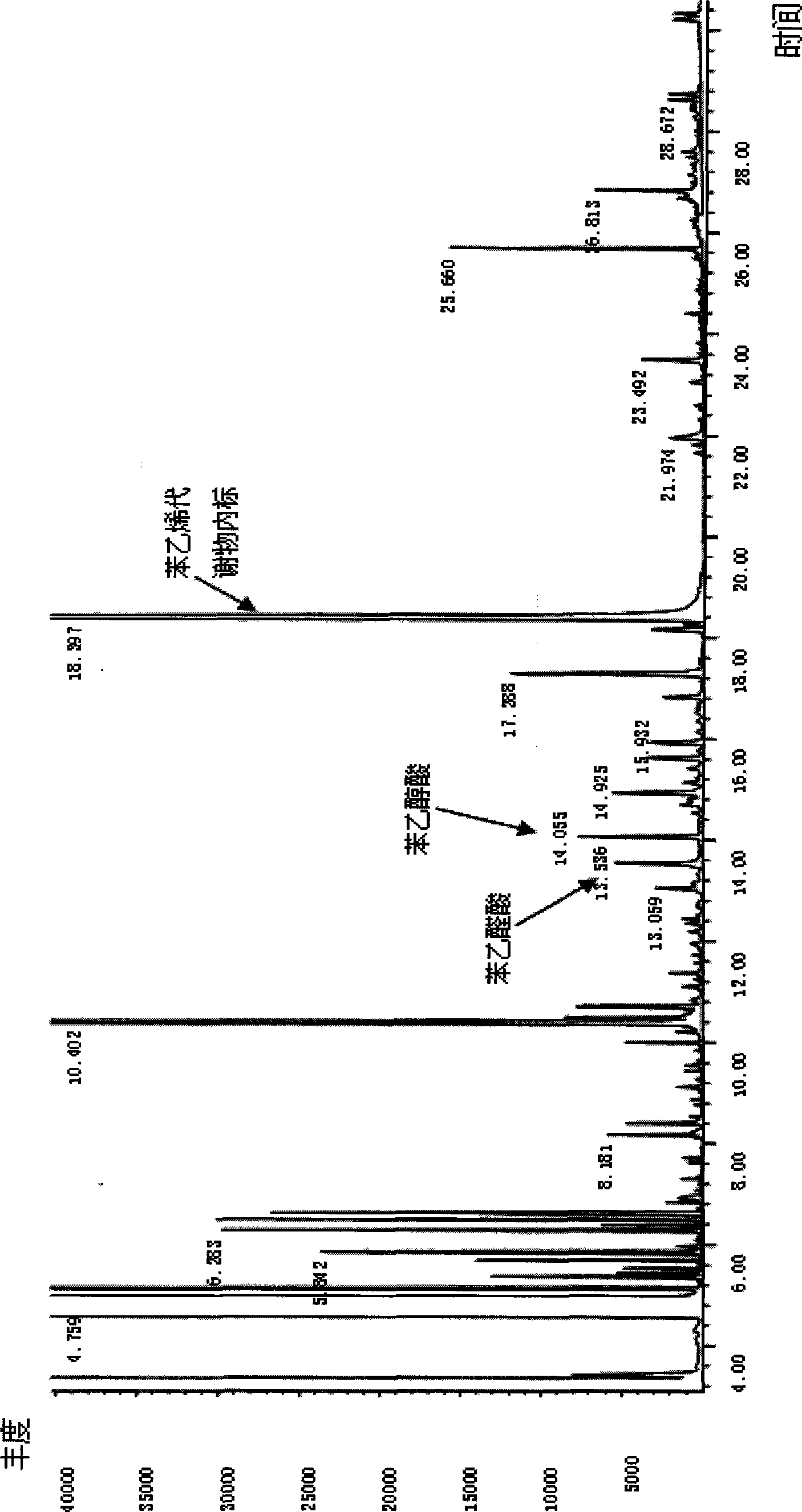
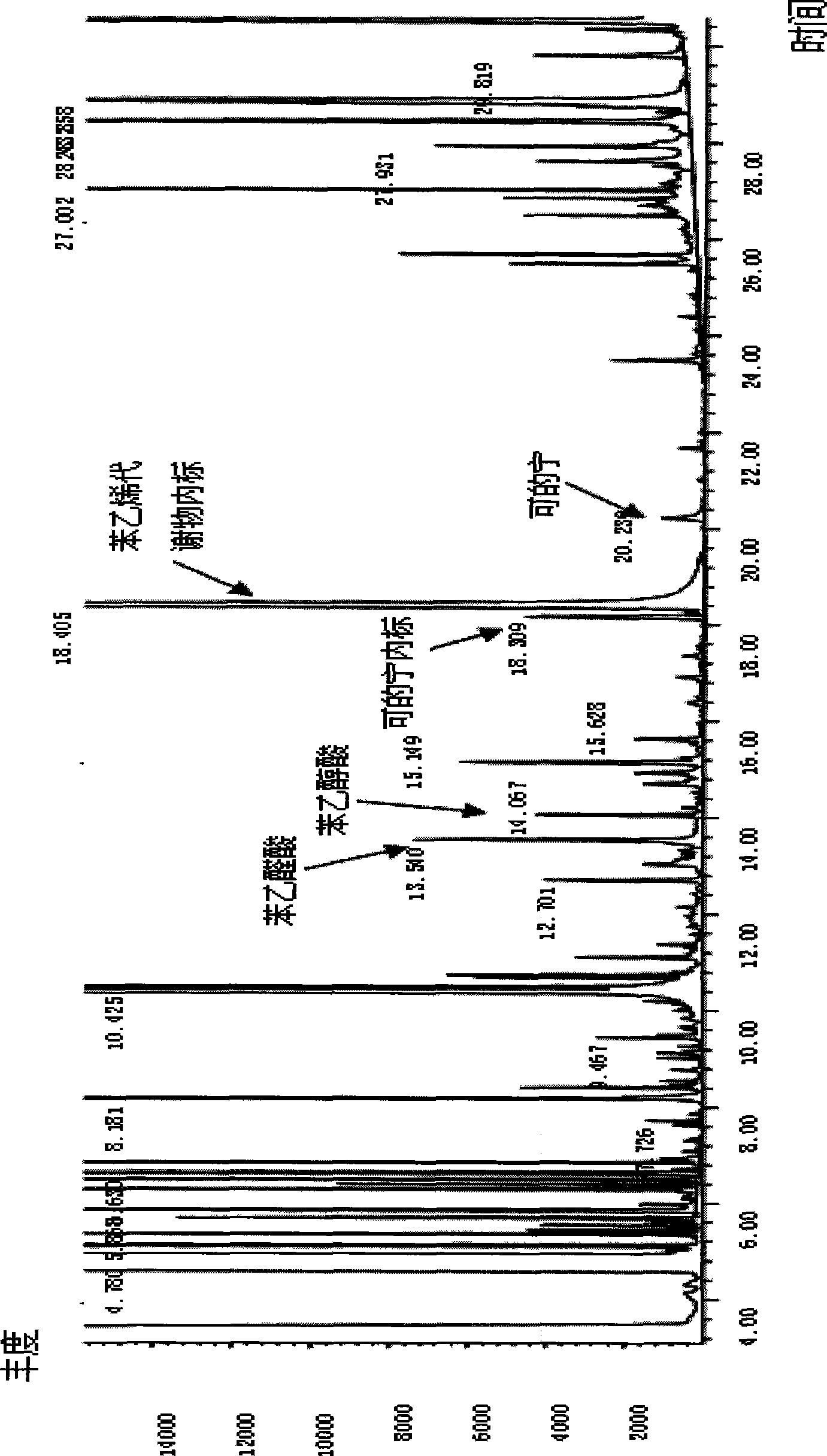
[0039]Take 3 urine samples stored at -20°C, marked as No. 1, 2, and 3 respectively, centrifuge after natural thawing, and carry out the following experimental procedures at the same time: Take 1ml of supernatant urine sample, add 5ul of 2mg / ml benzophenone, Add 50ul of 2mg / ml diphenylamine, add 100ul of 1.2M HCL, extract with 1ml of chloroform, vortex at 1500rpm for 5min, then centrifuge at 2500rpm for 4min, then take the organic phase A; Chloroform was used for the second extraction, vortexed at 1500rpm for 5min, centrifuged at 2500rpm for 4min, and the organic phase B was taken. Mix the two organic phases A and B in the same glass tube, blow dry; then add 50ul acetonitrile to redissolve, add 50ul derivatization reagent MSTFA, derivatize at 50°C for 30min. Take 1ul of the reaction solution for gas chromatography-mass spectrometry determination; GC / MS conditions are: DB-5ms column, EI ion source, voltage 70eV, ion source temperature 230°C, inlet temperature 270°C; SIM method ...
Embodiment 2
[0041] After adjusting the diet and living habits of Person No. 3, take the urine sample of Person No. 3 again, and carry out the following experimental steps: Take 1ml of supernatant urine sample, add 5ul 2mg / ml benzophenone, 50ul 2mg / ml diphenone Aniline, add 100ul 1.2M HCL, extract with 1ml chloroform, vortex at 1500rpm for 5min, then centrifuge at 2500rpm for 4min, then take the organic phase A; add 130ul 1M sodium hydroxide to the remaining liquid, and use 1ml chloroform for the second For secondary extraction, vortex at 1500rpm for 5min, then centrifuge at 2500rpm for 4min, and take the organic phase B. Mix the two organic phases A and B in the same glass tube, blow dry; then add 50ul of acetonitrile to redissolve, add 50ul of derivatization reagent MSTFA, derivatize for 30min at 50°C; take 1ul of the reaction solution for gas chromatography-mass spectrometry . The GC / MS conditions are as follows: DB-5ms column, EI ion source, voltage 70eV, ion source temperature 230°C,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com