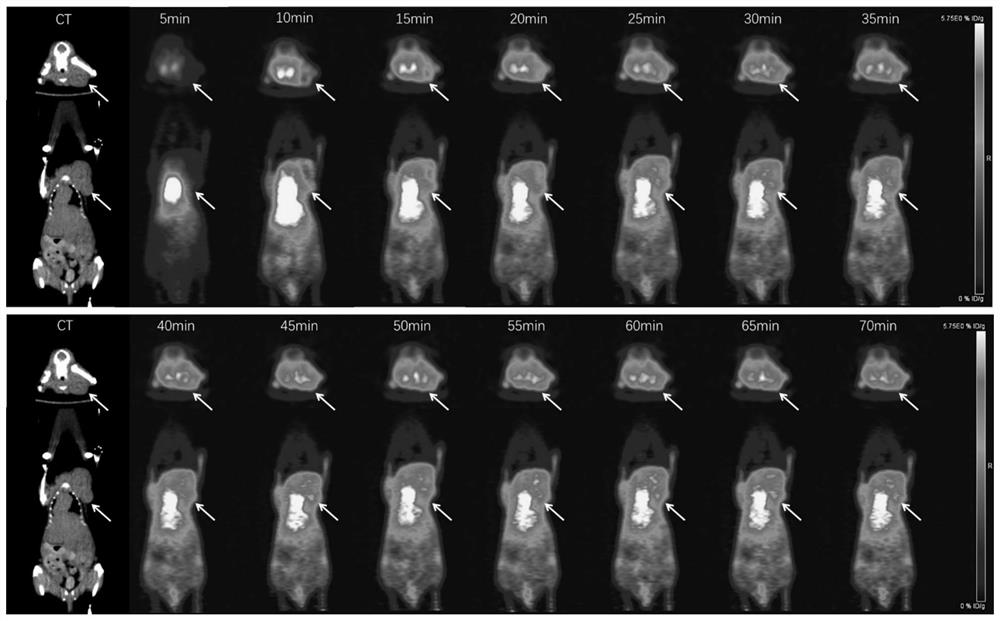
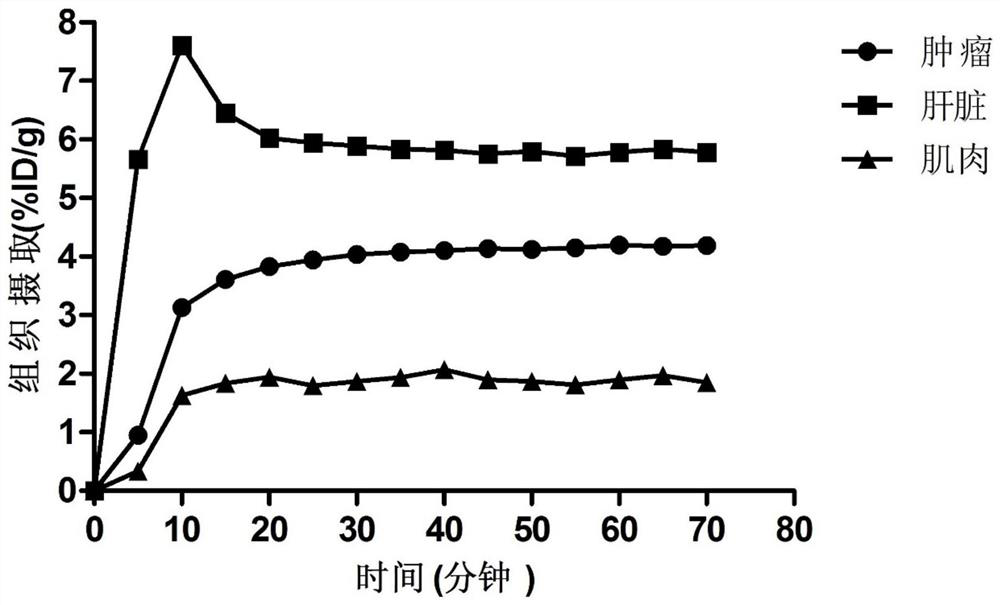
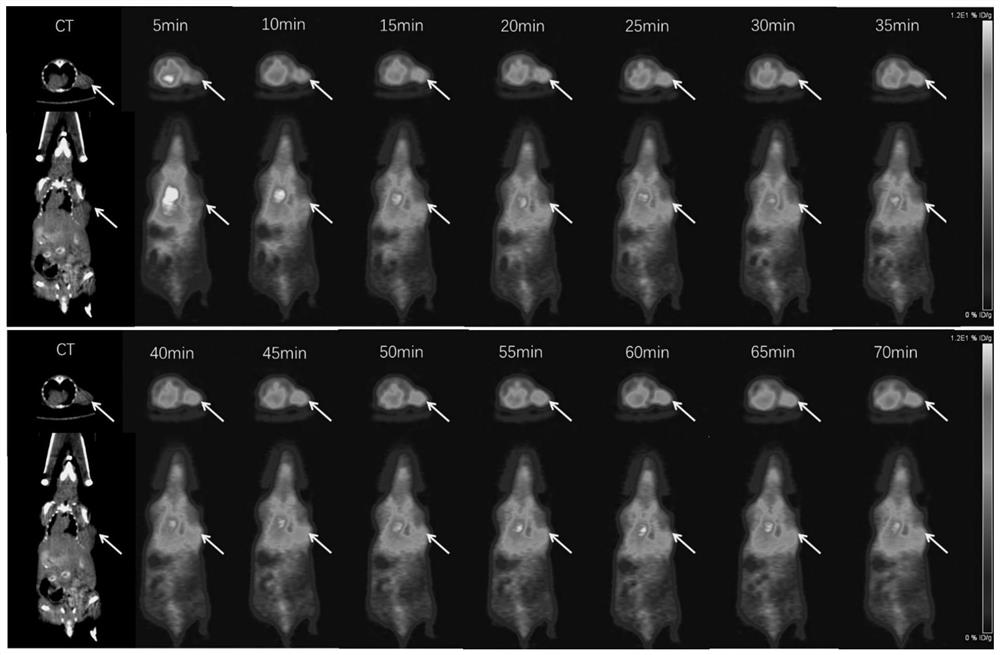
Radioisotope labeled polypeptide developer of targeted transferrin receptor and application of radioisotope labeled polypeptide developer
A technology of radioisotopes and transferrin, which is applied in the field of radioisotope-labeled polypeptide imaging agents, can solve the problems of long distribution time, short elimination half-life, and functional inactivation, achieving high accuracy and sensitivity, simple labeling methods, and labeling high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0093] Preparation of polypeptide precursors
[0094] 1. Preparation of Fmoc-Pip-OH
[0095] Fmoc-Pip-OH can be obtained by protecting 4-amino-(1-carboxymethyl)piperidine with tert-butanol and Fmoc-Cl, of course, it can also be prepared by other methods familiar to those skilled in the art.
[0096] The structural formula of Fmoc-Pip-OH finally prepared in this embodiment is as follows:
[0097]
[0098] 2. Preparation of Fmoc-AEEP-OH
[0099] Fmoc-AEEP-OH can be obtained by sequential protection of 3-[2-(2-aminoethoxy)ethoxy]-propionic acid through tert-butanol and Fmoc-Cl. Of course, other methods familiar to those skilled in the art can also be used. method to prepare.
[0100] The structural formula of Fmoc-AEEP-OH finally prepared in this embodiment is as follows:
[0101]
[0102] 3. Preparation of NOTA-tBu(2)
[0103] NOTA-tBu (2) can be prepared by reacting NOTA and tert-butanol under the trifluoromethanesulfonic anhydride catalyzed system, and can also be p...
Embodiment 168
[0121] Example 1 68 Preparation of Ga-NOTA-Pip-HAIYPRH
[0122] 1. 68 Ga-labeled NOTA-Pip-HAIYPRH
[0123] Dissolve 20ug of the above-mentioned polypeptide precursor NOTA-Pip-HAIYPRH in 200ul 1M sodium acetate solution, put it into a reaction bottle, and draw 0.05mol / L HCl 5ml with a 5ml syringe to 68 Ge / 68 The Ga generator performs segmental leaching, the flow rate is 1ml / min, and the middle 1.5ml of high activity is collected 68 Add the Ga eluent into the reaction bottle, adjust the pH of the reaction solution to 3.8-4.2, seal it, and place it in a water bath with a multifunctional heater at 90°C for 10 minutes.
[0124] The radiochemical purity of the product was measured, and if the radiochemical purity of the product was ≥80%, it indicated that the labeling was successful.
[0125] 2. Product purification
[0126] Draw the above-mentioned labeled product solution after the reaction with a syringe, add it to the activated Sep-pak C-18 column, and then use 5ml physio...
Embodiment 268G
[0131] Example 2 68 Preparation of Ga-NOTA-AEEP-HAIYPRH
[0132] 1. 68 Ga-labeled NOTA-AEEP-HAIYPRH
[0133] Dissolve 20ug of the above-mentioned polypeptide precursor NOTA-AEEP-HAIYPRH in 200ul 1M sodium acetate solution, put it into a reaction bottle, and draw 0.05mol / L HCl 5ml with a 5ml syringe to 68 Ge / 68 The Ga generator performs segmental leaching, the flow rate is 1ml / min, and the middle 1.5ml of high activity is collected 68 Add the Ga eluent into the reaction bottle, adjust the pH of the reaction solution to 3.8-4.2, seal it, and place it in a water bath with a multifunctional heater at 90°C for 10 minutes.
[0134] The radiochemical purity of the product was measured, and if the radiochemical purity of the product was ≥80%, it indicated that the labeling was successful.
[0135] 2. Product purification
[0136] Draw the above-mentioned labeled product solution after the reaction with a syringe, add it to the activated Sep-pak C-18 column, and then use 5ml phy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com