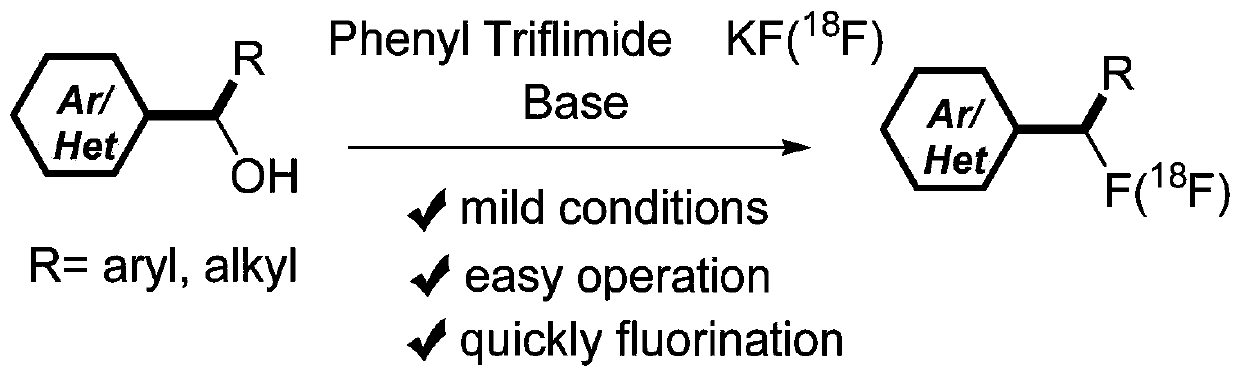
Alcohol compound-based in-situ deoxygenation fluorination synthesis method and alcohol compound-based <18>F radiolabeling method
A technology of radioactive labeling and synthesis method, which is applied in the preparation of organic compounds, steroid compounds, organic chemical methods, etc., to achieve the effects of easy realization, wide substrate applicability, simple and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
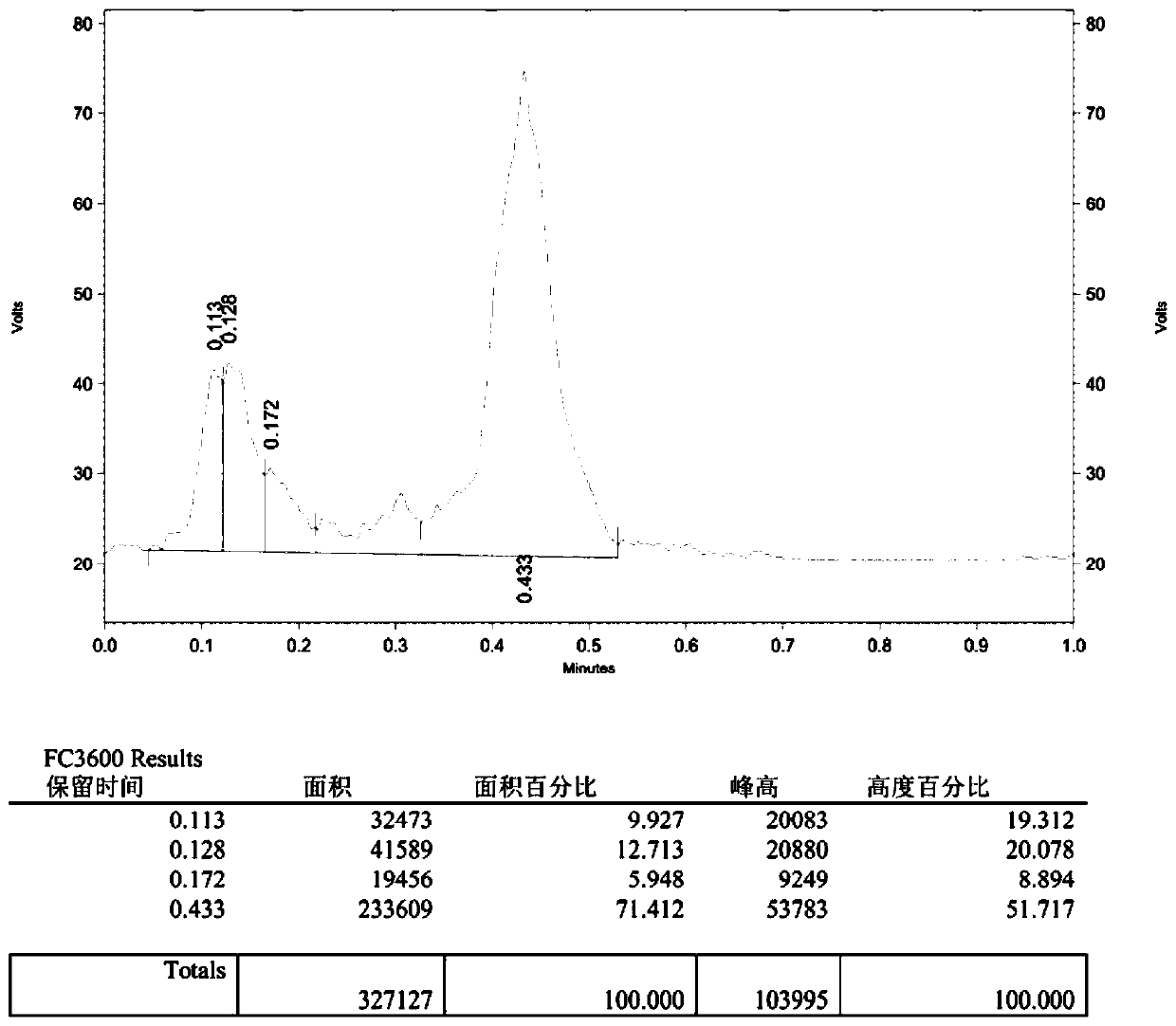
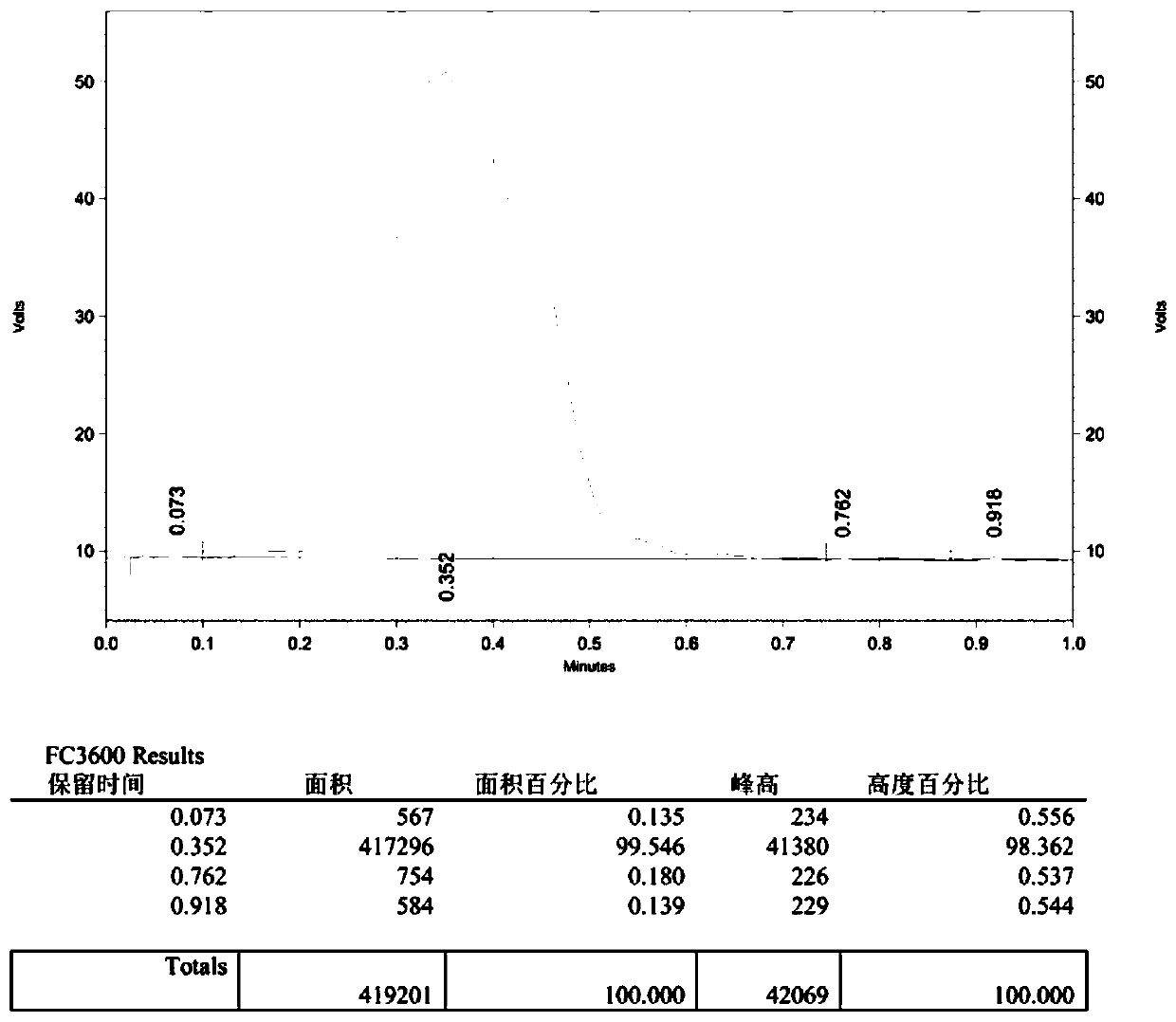
Image
Examples
Embodiment 1
[0044]
[0045] Add 4-phenyl-1-butanol (0.5 mmol), MTBD (7-methyl-1,5,7-triazabicyclo[4.4.0 ]dec-5-ene) (0.75mmol), then add 2.5mL tetrahydrofuran; in another reaction tube 2 equipped with a magnetic stirrer 5ml, add N-phenylbis(trifluoromethanesulfonyl)imide (2.5mmol ) and potassium fluoride (3.75mmol), then add 2mL of N,N-dimethylformamide, the two tubes are connected by a catheter, the reaction tubes 1 and 2 are fixed on the oil bath, and stirred. After the reaction system was reacted at 60°C for 60 minutes, the reaction was completed; add an appropriate amount of water to the reaction solution in reaction tube 1, extract with ethyl acetate, dry with anhydrous sodium sulfate, and finally remove the solvent by rotary evaporation, and the crude product was passed through the column layer Analysis (petroleum ether) separation and purification to obtain the target product (2a), with a yield of 95%. The NMR data of this compound are: 1 H NMR (400MHz, CDCl 3 )δ7.30(dd, J=9....
Embodiment 2
[0047]
[0048] Replace 4-phenyl-1-butanol (1a) with 3-(4-bromo-phenyl)propanol (1b), others are the same as Example 1, and column chromatography (petroleum ether) obtains target product (2b), Yield 78%. The NMR data of this compound are: 1 H NMR (600MHz, CDCl 3 )δ7.42(dd, J=8.1,1.3Hz,2H),7.08(d,J=6.9Hz,2H),4.48(td,J=5.7,1.4Hz,1H),4.41(td,J=5.8 ,1.4Hz,1H),2.71(t,J=7.1Hz,2H),2.04–1.90(m,2H). 13 C NMR (101MHz, CDCl 3 )δ140.05(s), 131.53(s), 130.26(s), 119.82(s), 83.67(s), 82.02(s), 31.86(d, J=19.8Hz), 30.78(d, J=5.2 Hz). 19 F NMR (564MHz, CDCl 3 )δ-218.77–-220.57(m).
Embodiment 3
[0050]
[0051] Replace 4-phenyl-1-butanol (1a) with 4-biphenylmethanol (1c), others are the same as Example 1, and column chromatography (petroleum ether: ethyl acetate=100:1) obtains target product (2c ), yield 51%. The NMR data of this compound are: 1 H NMR (600MHz, CDCl 3 )δ7.61(dd, J=16.1,7.6Hz,4H),7.45(t,J=7.7Hz,4H),7.36(t,J=7.4Hz,1H),5.46(s,1H),5.38( s, 1H). 13 C NMR (101MHz, CDCl 3 )δ141.77(d, J=3.2Hz), 140.62(s), 128.84(s), 128.07(d, J=5.7Hz), 127.65–127.27(m), 127.17(s), 85.24(s), 83.59(s). 19 F NMR (564MHz, CDCl 3 )δ-206.07-206.24(t).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com