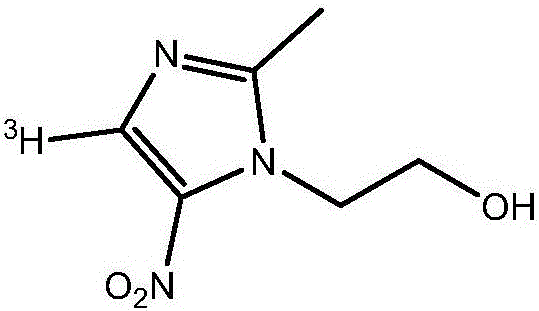
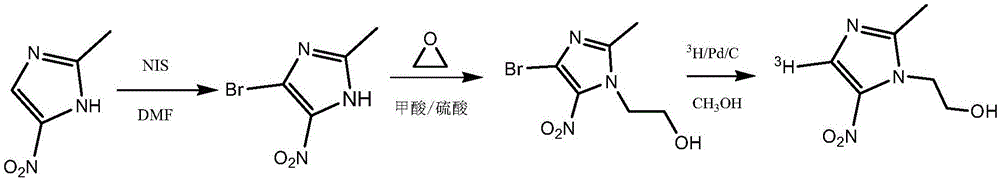
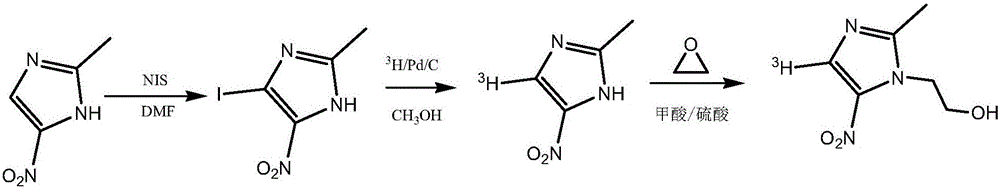
Tritiated metronidazole and preparation method thereof
A metronidazole and labeling technology, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of lack of metronidazole metabolism, residue and elimination research, impact on food safety monitoring of human health, residue exceeding the standard, etc., to achieve less environmental pollution , easy to control, high specific activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Add 6.35 g of 2-methyl-5-nitroimidazole and 50 ml of N,N-dimethylformamide in sequence in a reaction vessel with a stirring device and equipped with a thermometer, stir and dissolve the raw materials at 40°C, and divide Add 11.25 g of N-iodosuccinimide in batches, react at 80°C for 5 hours, cool the reaction solution to 20°C, add an equal volume of ice water to the reaction solution, filter to obtain a yellow solid, and use 10% Sodium thiosulfate aqueous solution and refrigerated distilled water were washed, the obtained solid was filtered, recrystallized with ethanol, and vacuum-dried to obtain 4-iodo-2-methyl-5-nitroimidazole in the form of light yellow crystals with a yield of 65%.
[0034] (2) Add 6.35g of 2-methyl-5-nitroimidazole and 60ml of N,N-dimethylformamide successively into a reaction vessel equipped with a thermometer equipped with a stirring device, stir and dissolve the raw materials at 40°C, and divide Add 16.87 g of N-iodosuccinimide in batches, re...
Embodiment 2
[0037] (1) In the 5mL micro-reaction bottle, add magneton successively, then add 50 mg of 4-bromo-2-methyl-5-nitroimidazole prepared in Example 1, add 3 mL of methanol to completely dissolve it, and then add 5 mg of 10% Concentration of palladium carbon (Pd / C) as catalyst. The reaction bottle was placed on a hydrogenation device and fed with high-purity hydrogen gas, replaced three times, the reaction pressure was adjusted to 100mmHg, and the reaction was kept at 25°C for 30 minutes with stirring. The reaction was stopped, Pd / C was removed by centrifugation, and the remaining liquid was evaporated to dryness under reduced pressure to obtain 2-methyl-5-nitroimidazole, which was analyzed by HPLC with a yield of 47%.
[0038] (2) Add magneton successively in 5mL micro reaction vial, then add 4-bromo-2-methyl-5-nitroimidazole 50mg prepared in Example 1, add 3mL methanol to make it dissolve completely, then add 2.5mg 10 % concentration of Pd / C. The reaction bottle was placed on a...
Embodiment 3
[0044] (1) Add 100 mg of formic acid and 20 mg of concentrated sulfuric acid to a reaction vessel equipped with a thermometer, a stirrer and an air duct respectively to prepare a mixed acid solution, add 25 mg of 2-methyl-5-nitroimidazole to the mixed acid solution, and take another A two-necked reaction flask, add 5ml of ethylene oxide to connect the reaction system, slowly introduce ethylene oxide into the reaction vessel, react at 80°C for 12 hours, add an appropriate amount of 1M sodium hydroxide solution to the reaction solution to adjust the pH To 8, add 5ml of ethyl acetate solvent extraction three times, combine the organic phases, dry with anhydrous sodium sulfate and filter, distill the filtrate to remove the solvent under reduced pressure to obtain 29.5 mg of metronidazole with a yield of 88%.
[0045] (2) Add 75 mg of formic acid and 15 mg of concentrated sulfuric acid to a reaction vessel equipped with a thermometer, a stirrer and an air duct respectively to prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com