Combination therapy of an anti cd20 antibody with a btk inhibitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
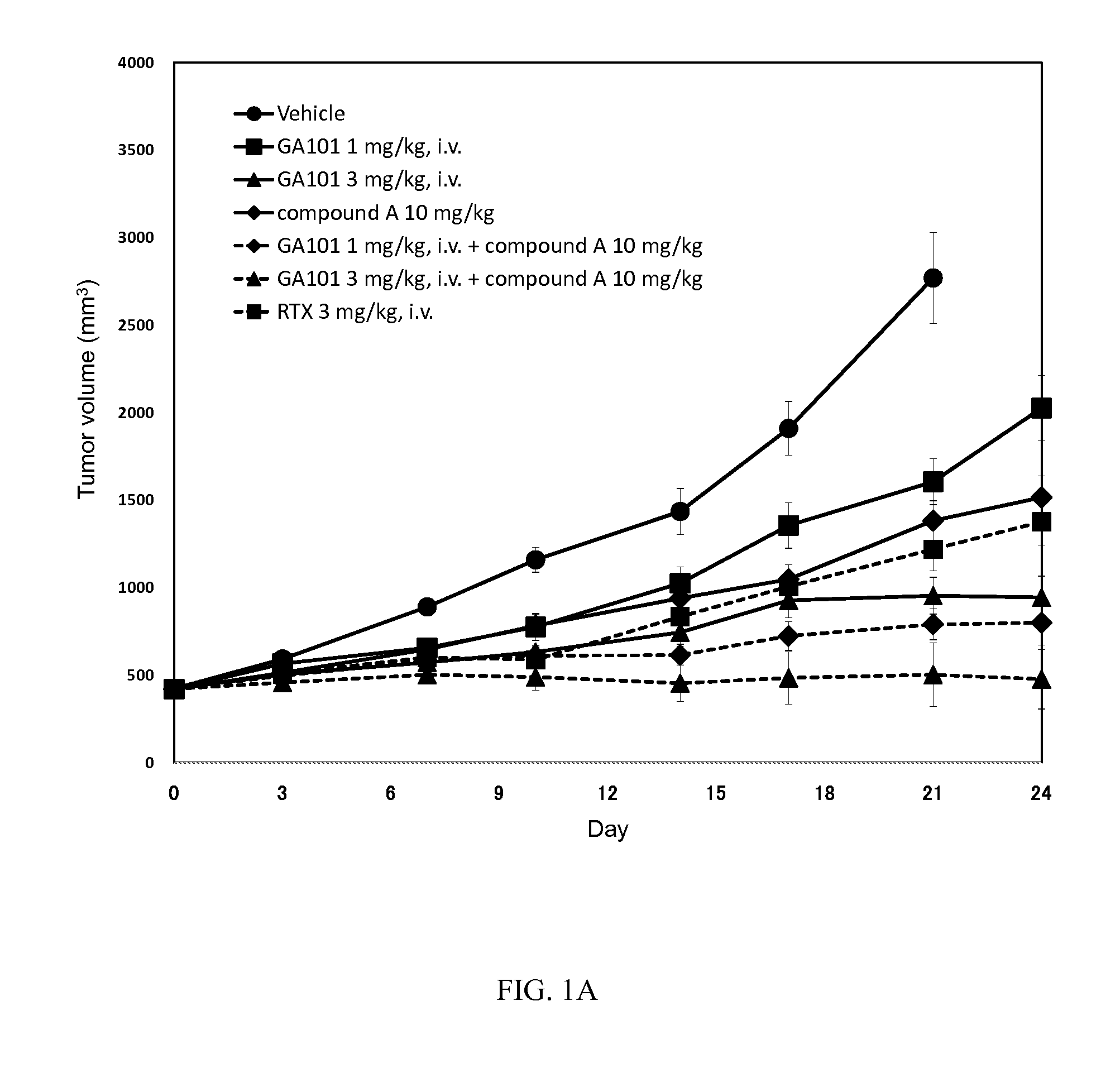
[0157]Antitumor efficacy study of BTK inhibitor in combination with anti-CD20 Abs in SCID mice subcutaneously xenografted with TMD8 (ABC-DLBCL) cells
1. Materials and Methods
1.1. Test Substances
[0158]The test substance, 6-amino-9-[(3R)-1-(2-butynoyl)-3-pyrrolidinyl]-7-(4-phenoxyphenyl)-7,9-dihydro-8H-purin-8-one hydrochloride (hereinafter referred to as “compound A”), was stored in sealed containers at ambient temperature, protected from light.
[0159]GA101 (obinutuzumab, sold under the tradename Gazyva® or Gazyvaro®): 500 mg, Rituximab (RTX, sold under the tradenames Rituxan® and MabThera®): 200 mg, were supplied by Roche Glycart A G, and stored at 4° C.
1.2. Substance Vehicle
[0160]Compound A was weighed out and suspended in a 0.5 w / v % methyl cellulose 400 cP solution (Wako Pure Chemical Industries, Ltd., hereinafter referred to as “0.5% MC”), using a mortar and pestle, to obtain a 1 mg / mL suspension. The suspension was used within 7 days of preparation. The suspension was confirmed t...
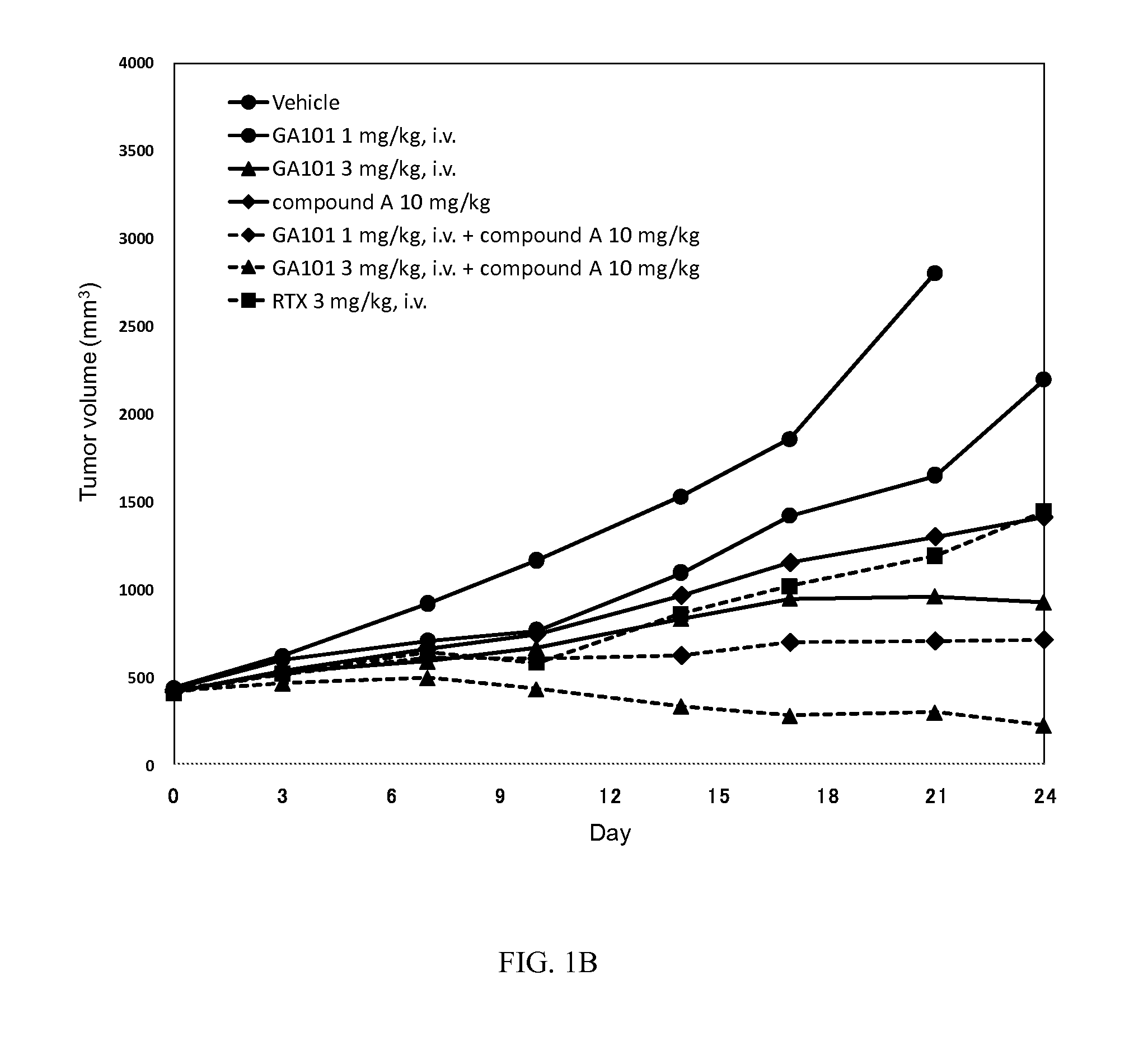
example 2
[0178]The experiment in Example 2 was performed according to the method described in Example 1 except for the following “substance vehicle” and “treatment schedule”.
1. 1. Substance Vehicle
[0179]Compound A was weighed out and suspended in a 0.5 w / v % methyl cellulose 400 cP solution (Wako Pure Chemical Industries, Ltd., hereinafter referred to as “0.5% MC”), using a mortar and pestle, to obtain a 1 mg / mL (10 mg / kg) suspension. The suspension was used within 7 days of preparation. The suspension was confirmed to be stable and uniform for 7 days under refrigeration, protected from light, and for a further 24 hours at ambient temperature exposed to indoor lighting.
[0180]The stock solutions of both GA101 (25 mg / mL) and Rituximab (10 mg / mL) were kept at 4° C. Before administration to mice, the stock solutions were diluted in NaCl 0.9% at 0.3 mg / mL (3 mg / kg) for this study.
1.2. Treatment Schedule
[0181]When the mean tumor volume reached 400 to 450 mm3 mice were randomized in groups of 6 acc...
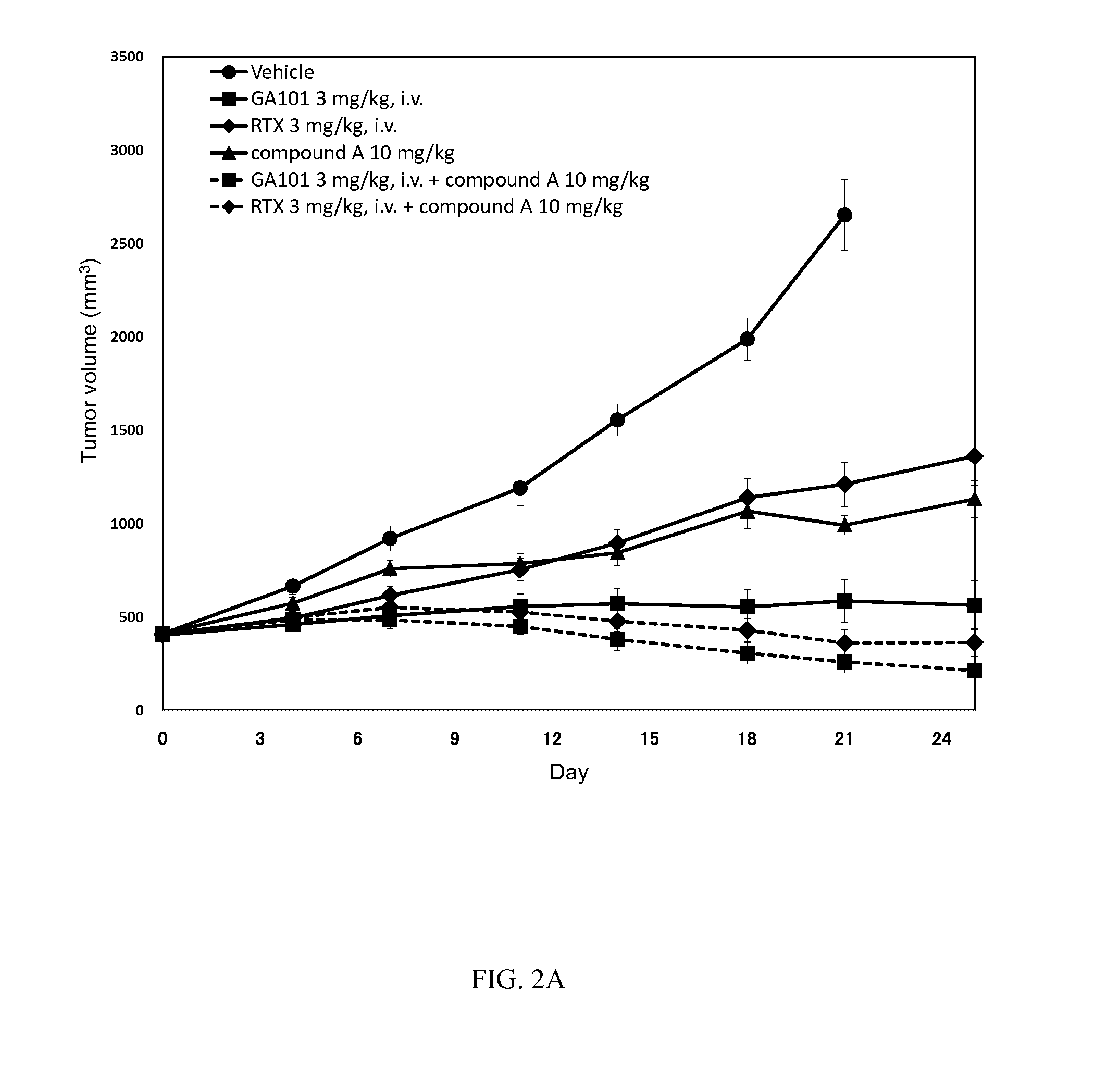
example 3
[0187]The experiment in Example 3 was performed according to the method described in Example 1 except for the following “substance vehicle”, “route of drug administration” and “treatment schedule”
1.1 Test Substance
[0188]The comparative substance, ibrutinib (hereinafter referred to as “compound B”), was stored in sealed containers at ambient temperature, protected from light.
1.2 Route of Drug Administration
[0189]Mice were fed a commercial diet (CRF1, Oriental Yeast Co., Ltd.) supplemented with either 0.0037% (wt / wt incorporated in the pellets) of compound A or 0.012% (wt / wt incorporated in the pellets) of compound B, respectively. The dose of compound A or compound B corresponds to a daily intake of 6 or 20 mg / kg of body weight. This calculation was based on the mean daily food intakes ranged from 160 to 180 mg / g of body weight.
[0190]Two groups (vehicle and RTX): Mice fed a normal diet (CRF1). Treatment groups: Mice fed the same diet containing compound A or com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Cytotoxicity | aaaaa | aaaaa |
| Chemotherapeutic properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com