Generation of heavy-chain only antibodies in transgenic animals
a technology of transgenic animals and antibodies, applied in the field of transgenic animal generation of only heavy chain antibodies, can solve the problems of limited antibody response repertoire, laborious use of camelid vsub>h /sub>regions, and inability to generate camelid vsub>h /sub>, so as to maximize the number of vh regions, increase antibody diversity, and maximize the effect of vh regions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A Heavy Chain-Only Antibody Locus is Fully Functional in Mice and Sensitive to Allelic Exclusion
[0102]As discussed above, Janssens et al. [15] have developed methods for the derivation of heavy chain-only antibodies in transgenic mice. For further details of the methods and experiments described herein, the skilled person should refer to Janssens et al. [15], which is incorporated herein by reference.1
Methods
Constructs
[0103]A genomic cosmid library was made from peripheral blood cells of Lama Glama using standard methods. Two different germline VHHs were chosen based on their sequence, an open reading frame without stop codon and the presence of hydrophilic amino acid codons at positions 42, 50 and 52 according to Lefranc numbering [32] and one with and one without a hydrophilic amino acid at position 49. One is identical to IGHV1S1 (acc.num. AF305944) and the other has 94% identity with IGHV1S3 (acc. num.AF305946). Two clones were selected from the human genomic Pac library RPCI-1...
example 2
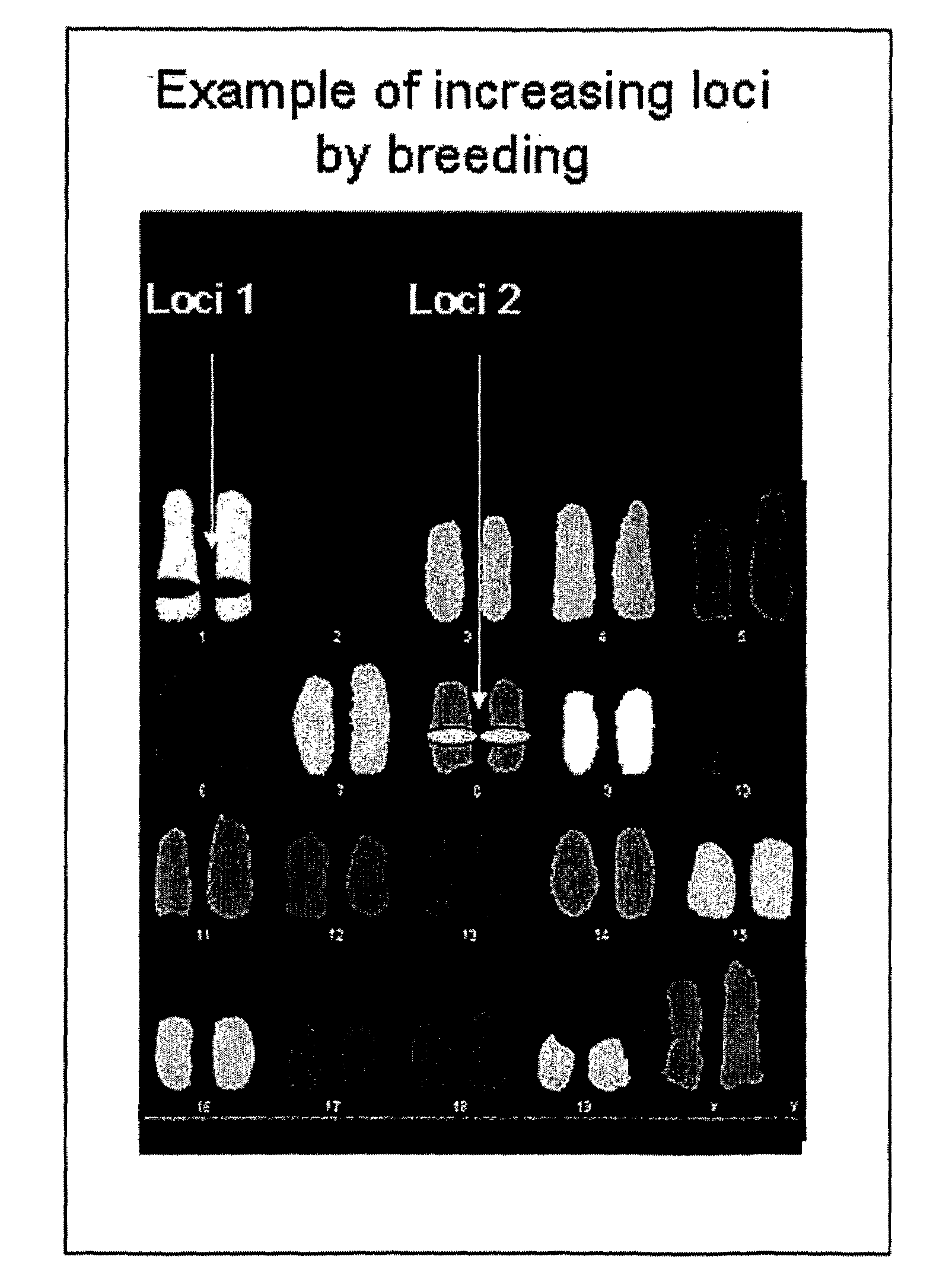
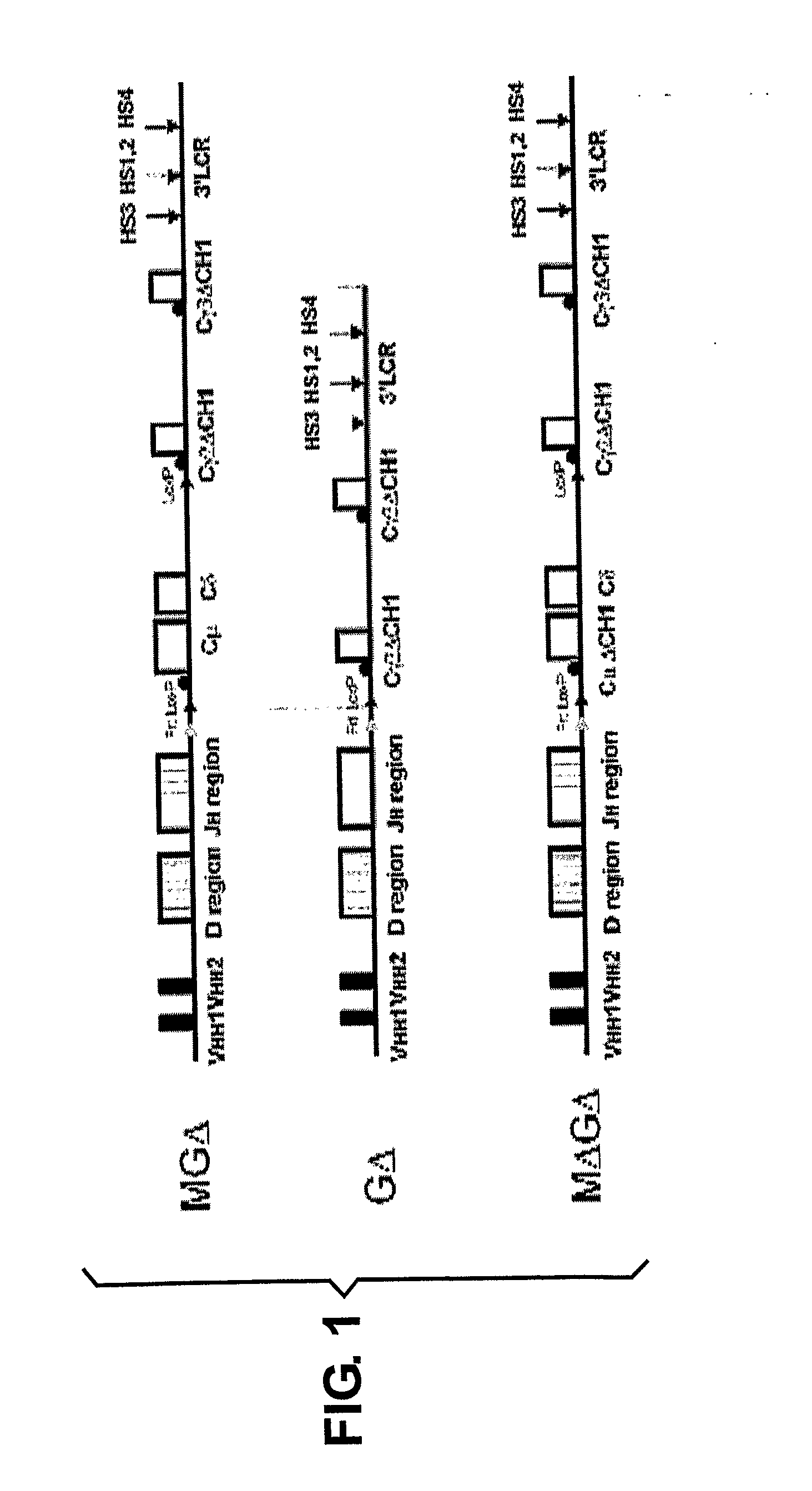
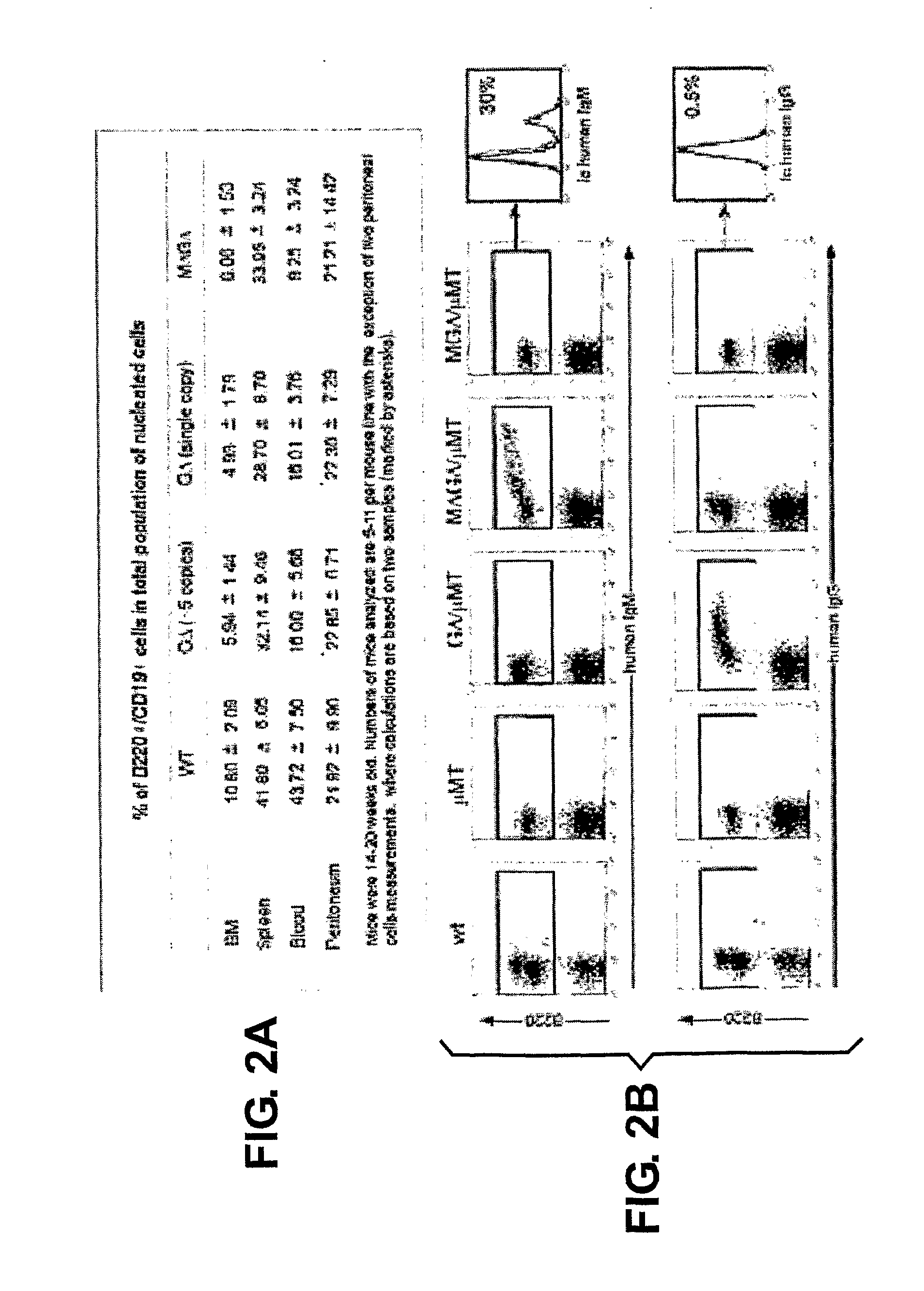
[0148]Janssens et al. (2006) have shown that a transgenic VH locus recombines properly to produce transgenically coded heavy chain only antibodies and that such a locus is sensitive to allelic exclusion in the presence of the endogenous (mouse) heavy chain immunoglobulin locus. In order to show that the number of VH transgenic loci, that is used for heavy chain only antibody production, can be increased by using the process of allelic exclusion, two transgenic mice containing different heavy chain only loci were crossed resulting in offspring containing both loci. One mouse contained the MGΔ locus (IgM and IgG locus, Janssens et al., 2006), while the other contained the GΔ locus (IgG locus only, Janssens et al., 2006), both mice have the μMT background. Hybridomas were derived from the B cells from the double transgenic offspring and grown in culture by standard methods. A number of the resulting individual monoclonal cell lines were analysed by PCR and Southern blots, which showed ...
examples 3 and 4
[0149]In a preferred embodiment of the first aspect of the invention all of the number of functional human VH regions is increased by cloning human VH regions (or variants thereof) onto a multiple modified human locus containing the entire DH region, the entire JH region and a combination of the Cμ, Cγ2, Cγ3 and Cα regions and the 3′LCR using those methods described in the previous example and known in the art (Janssens et al 2006). This procedure can be carried out using multiple identical VH regions on separate loci or different VH region on separate loci. The different loci can contain identical heavy chain regions or different heavy chain regions. The example 3 is for a locus with identical VH regions on loci that have a different combination of heavy chain regions, example 4 for two loci with identical heavy chain constant regions but different VH regions. Obviously in both examples additional loci could be added.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| solubility properties | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com