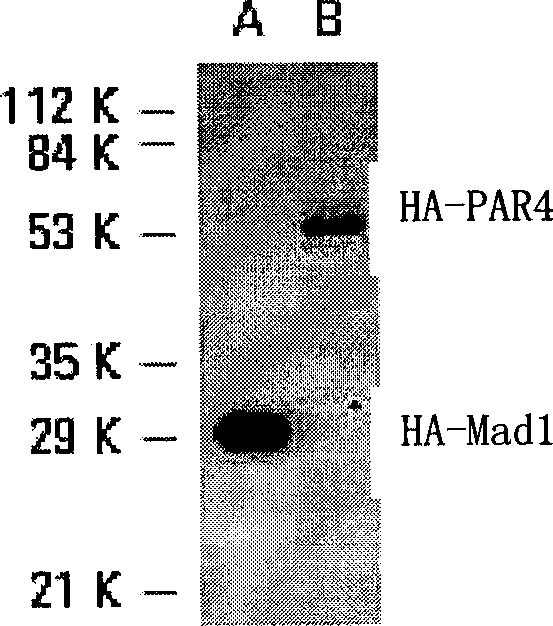
Preparation, identification and application of HA tag monoclonal antibody
A monoclonal antibody and labeling technology, applied in the field of biomedicine, can solve problems such as buried in the fusion target protein, increased protein purification rate, and protein aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] 1. Materials:
[0022] HA hexapeptide was synthesized by GenScript Company; ovalbumin (OVA), bovine serum albumin (BSA), rabbit anti-mouse IgA, IgG1, IgG2a, IgG2b, IgG3 and IgM antibodies were purchased from Sigma Company; HRP-labeled goat anti-mouse IgG was provided by Santa Cruz; MULTISKAN MK3 microplate reader was purchased from Thermo; 3326 CO 2 The incubator was purchased from Forma Company; the nitrocellulose membrane (NC membrane) was purchased from Amersham Company; the SDS-PAGE electrophoresis instrument was purchased from BIO-RAD Company; BALB / c pure-line male mice (rats aged 6-8 weeks, body mass 18-22g) was purchased from the Institute of Zoology, Chinese Academy of Medical Sciences.
[0023] 2. Method
[0024] 2.1 Synthesis of artificial antigen
[0025] (1) Synthesis of immune antigen: Weigh 4.4mg HA-tag and 5mg OVA and dissolve in 1mL PB (0.05mol / L, pH7.0). After fully dissolved, add 200μL of PB solution containing 2.2mg EDC dropwise , mixed while drop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com