Activated coagulation detection reagent and application thereof
A detection reagent and coagulation technology, applied in the field of activated coagulation detection reagent, to achieve the effects of good effect, low cost and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 reagent
[0026] A kind of preparation of activated blood coagulation detection reagent is as follows:
[0027] Each component of described reagent is composed as follows:
[0028] Kaolin: 0.0024%
[0029] Mixed phospholipids: 20μg / mL
[0030] PVP40: 0.1%
[0032] Betaine: 5mM
[0033] Phenol: 0.36%
[0034]The percentages are mass volume percentages, and the unit is g / ml.
[0035] Calculate according to the total volume of 1L and accurately weigh or measure the corresponding above-mentioned components, disperse evenly with distilled water, and set the volume to 1L. Stir evenly and dispense into sample tubes, 40 μL each.
[0036] The preparation of mixed phospholipids is widely reported in professional literature (for example, Clin Chem.1997; 43(7):1215-1222.), and is not limited to specific and single specific conditions.
Embodiment 2
[0037] Embodiment 2 Detection effect verification of reagents
[0038] Detection of the effect of activated coagulation detection reagent
[0039] The activated coagulation detection reagent prepared in Example 1 and the imported activated coagulation reagent were tested on the TEG5000 analyzer with the same blood sample, and the parameters of the test were explained as follows: R represents the period between the detection and the formation of the first fibrin clot A period of time; Angle is used to evaluate the efficiency of blood clot formation; MA directly reflects the strongest dynamic characteristics of the interaction between fibrin and platelets through GPIIb / IIIa, representing the maximum strength of fibrin clot.
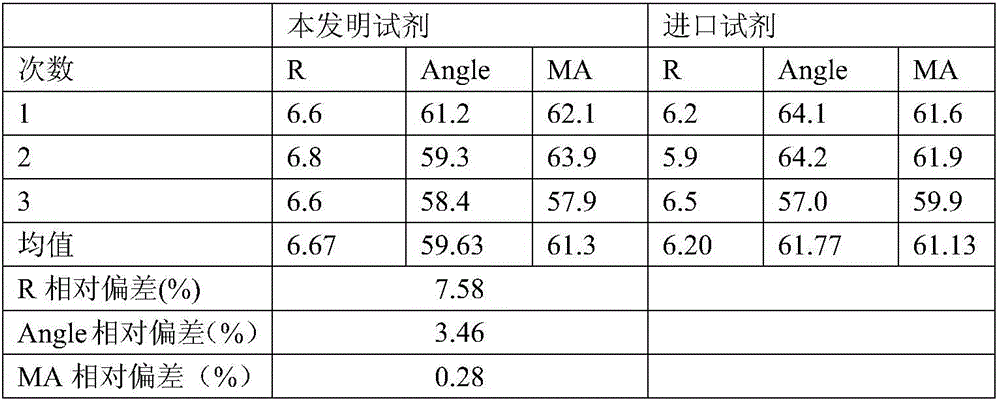
[0040] The detection data of embodiment 1 gained reagent and import reagent are as shown in table 1:
[0041] Table 1 Test results of various parameters
[0042]
[0043] As can be seen from Table 1, the reagents of the present invention are equivalent...
Embodiment 3
[0044] Embodiment 3 The stability research of reagent
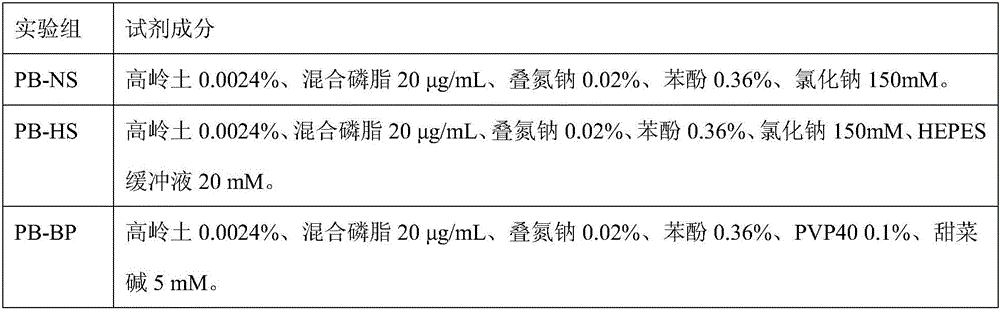
[0045] The accelerated stability of each group is detected respectively according to the test groups described in Table 2:
[0046] Table 2 Classification of experimental groups
[0047]
[0048] Note: The percentages in the above table are mass volume percentages, and the unit is g / ml.
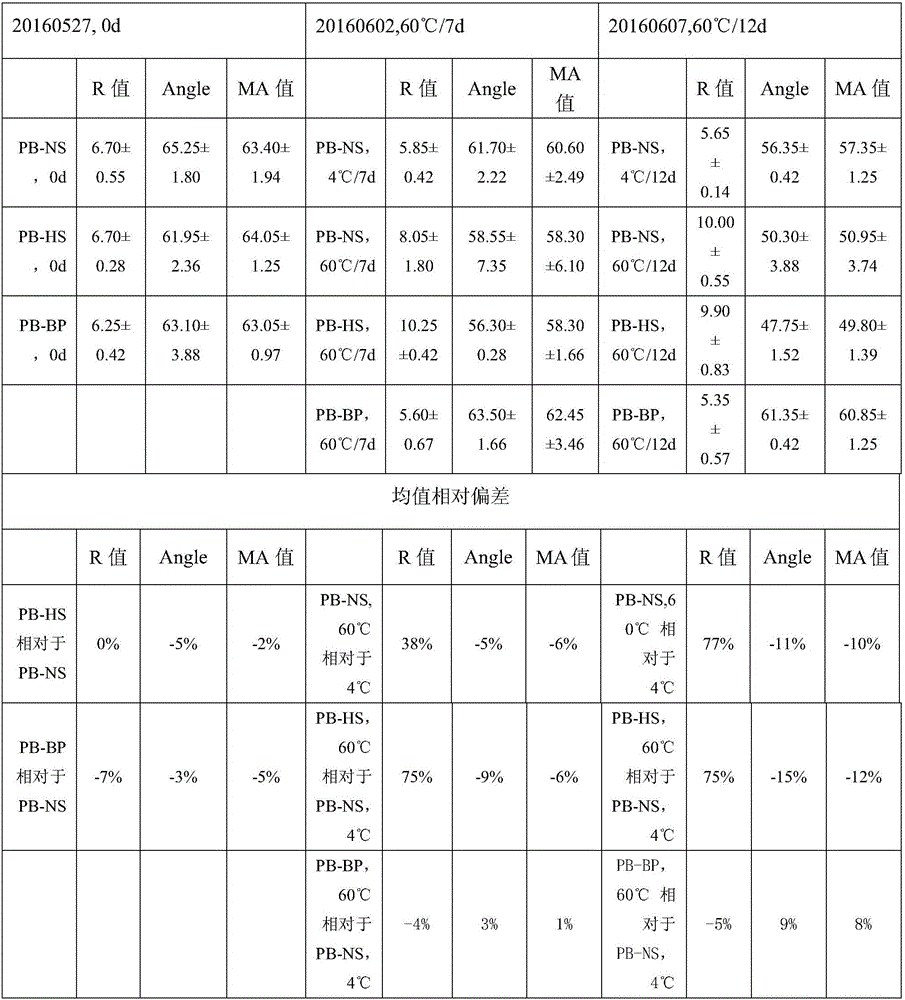
[0049] According to the preparation method described in Example 1, the three groups of reagents were formulated into reagent samples, and then each group of samples were stored at 60°C for accelerated destruction. The test was carried out on the first day, and the test results are shown in Table 3:
[0050] Table 3 represents the stability detection result of each group of reagents
[0051]
[0052] From the test data shown in Table 3, it can be seen that the test results of the three groups of reagents on the day of preparation are equivalent, and there is no significant difference in the test averages of R value, Angle value, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com