Method for constructing, expressing and purifying human recombination factor and application
A tissue factor and expression method technology, applied in chemical instruments and methods, biochemical equipment and methods, applications, etc., can solve the problems of low biological activity, reduced soluble protein expression level, low uniformity of TF structure and activity, etc. The effect of simplifying the process and improving the expression rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
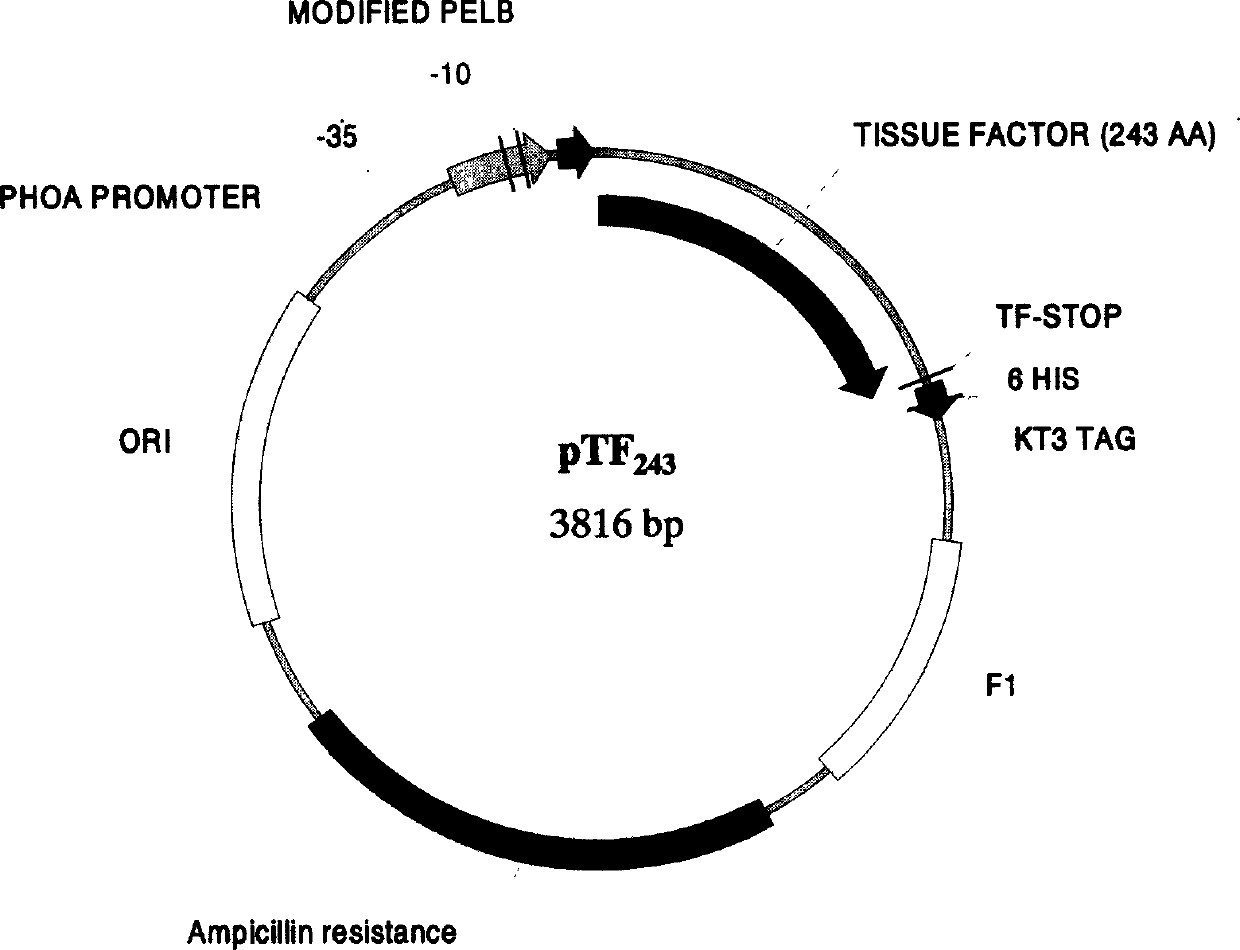
[0038] rhTF 243 Construction of expression plasmid vector
[0039] Total RNA was obtained from human placenta tissue, and PCR upstream primer P1 and downstream primer P2 were designed according to the known TF nucleotide sequence.
[0040] Upstream primer P1: 5'-ACCAGCCATGGCTCAGGCACTACAAATACTGTGGC-3'
[0041] Downstream primer P2: 5'-GGATCCTCAGTGTAGAGATATAGCCAGGATG-3'
[0042] The P1 primer contains several TF protein N-terminal amino acids and NcoI restriction sites, and the P2 primer contains several TF protein C-terminal amino acids, termination codes and BamHI restriction sites.
[0043] Using total RNA as a template and P1 and P2 as primers, the TF target gene fragment was obtained by conventional RT-PCR method; the collected rhTF 243 The gene fragment was treated with restriction enzymes NcoI and BamHI, and recombined with the phoA plasmid to construct the TF expression plasmid vector pTF 243 .
[0044] TF expression plasmid vector pTF 243 Transform into Escherichi...
Embodiment 2
[0046] rhTF 243 Fermentation expression of
[0047] 1. Preparation of fermentation medium
[0048] Composition: LB medium 1000mL, glucose 50g, inorganic salt solution 10mL, 0.01% ampicillin. The inorganic salt solution consists of 0.1% sodium dihydrogen phosphate, 0.5% dipotassium hydrogen phosphate, 0.01% ferric chloride, 0.03% zinc sulfate, 0.01% cobalt chloride, 0.03% copper sulfate, 0.5% ammonium sulfate and 0.05% magnesium sulfate composition.
[0049] After sterilizing the mixed solution of LB medium, glucose and inorganic salt at 121° C. for 20-30 minutes, ampicillin was added to prepare the fermentation medium.
[0050] 2. Shake flask fermentation culture
[0051] Take 2mL of the genetically recombined engineering bacteria liquid obtained by screening and add it to a conical flask containing 1000mL of fermentation medium, put it in a constant temperature shaker at 30°C, and cultivate it at a speed of 200r / min for 16h-20h, until the OD value at 600nm is measured at ...
Embodiment 3
[0059] rhTF 243 protein purification
[0060] 1. Preparation of TP monoclonal antibody immunoaffinity chromatography column
[0061] Weigh 15 mg of purified TF monoclonal antibody, 1 g of CNBr-activated Sepharose matrix, 100 mL of 0.4 mol / L NaHCO 3 Coupling buffer (pH8.3), 11.7 g NaCl, mixed at 25°C and packed into the column, shaken slowly for 2h, drained the liquid; then blocked with 0.1mol / L Tris buffer (pH8.2) for 2h, static at 25°C Set aside, drain the liquid; wash with 0.1 mol / L sodium acetate solution at pH 3.0 and 0.1 mol / L Tris buffer at pH 8.3 in sequence, and finally store with 5 times the column volume of PBS buffer at 4°C to prepare TP monoclonal antibody Immunoaffinity chromatography columns.
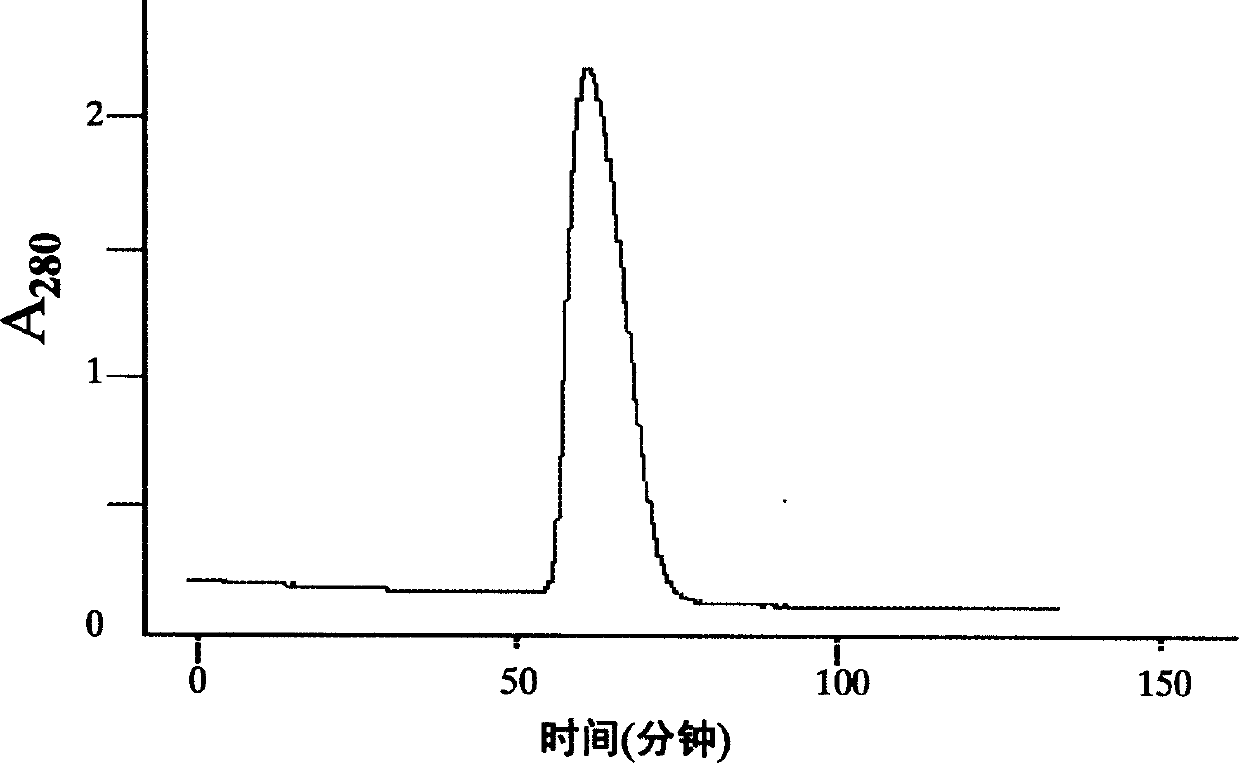
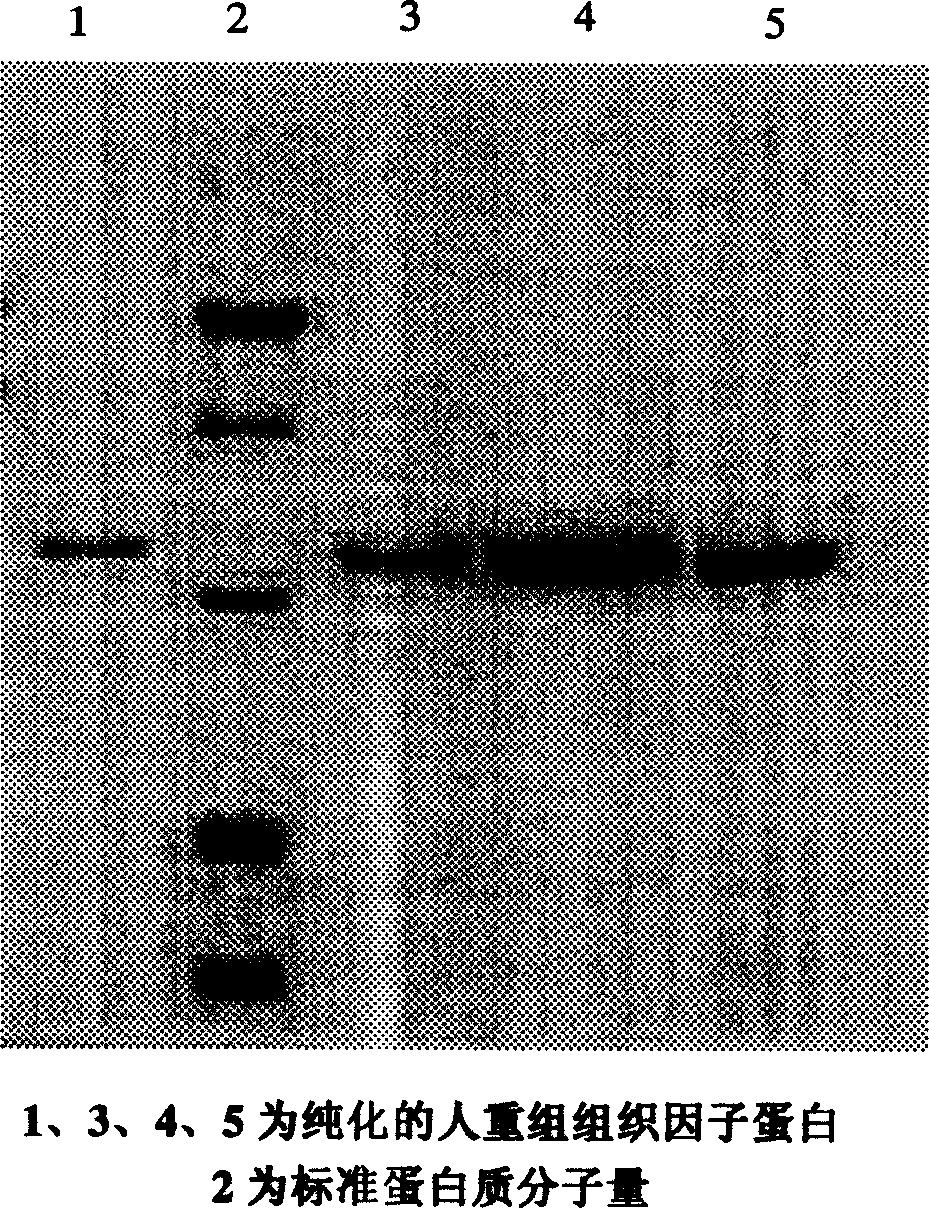
[0062] 2. rhTF 243 protein purification
[0063] 1) Bacterial fragmentation
[0064] Dissolve 20 g of the obtained fermented bacteria in 100 mL of 20 mmol / L Tris-EDTA buffer at 2 °C to 8 °C, add 1% Triton X-100, shake and mix, and use a high-pressure homogenizer to p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com