Method for inducing mesenchymal stem cells (MSCs) into chondrocytes
A technology of chondrocytes and mesenchymal stem cells, applied in the field of stem cells, can solve the problems of long cycle and low efficiency of type II collagen expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
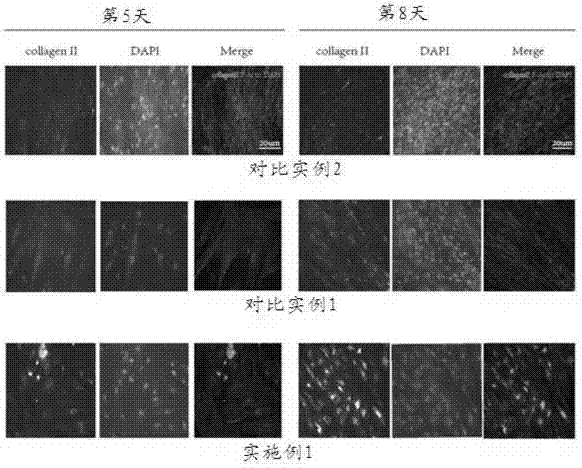
Examples
Embodiment 1
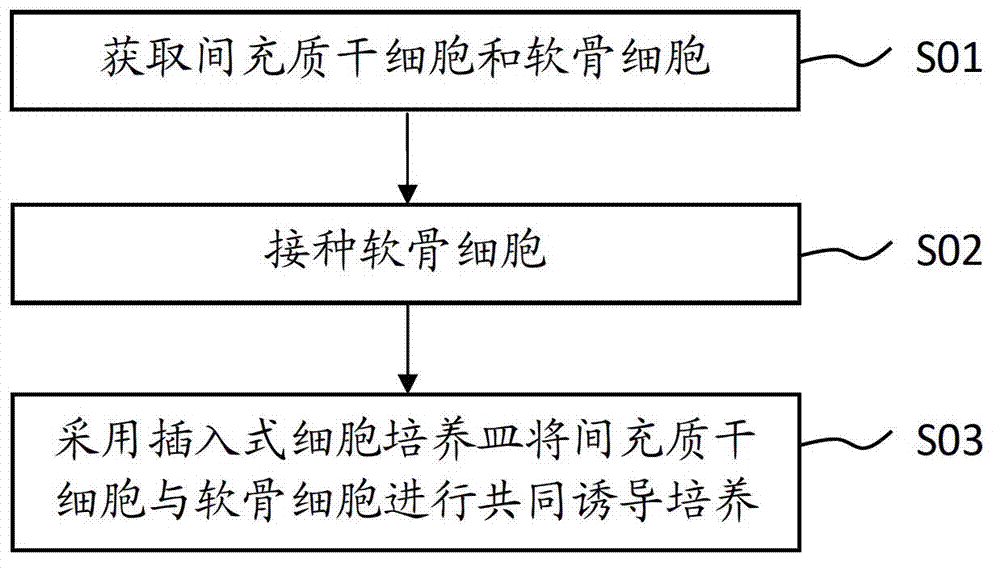
[0060] A method for inducing mesenchymal stem cells to become chondrocytes, comprising the steps of:
[0061] Step S11. Isolation and culture of umbilical cord MSCs:
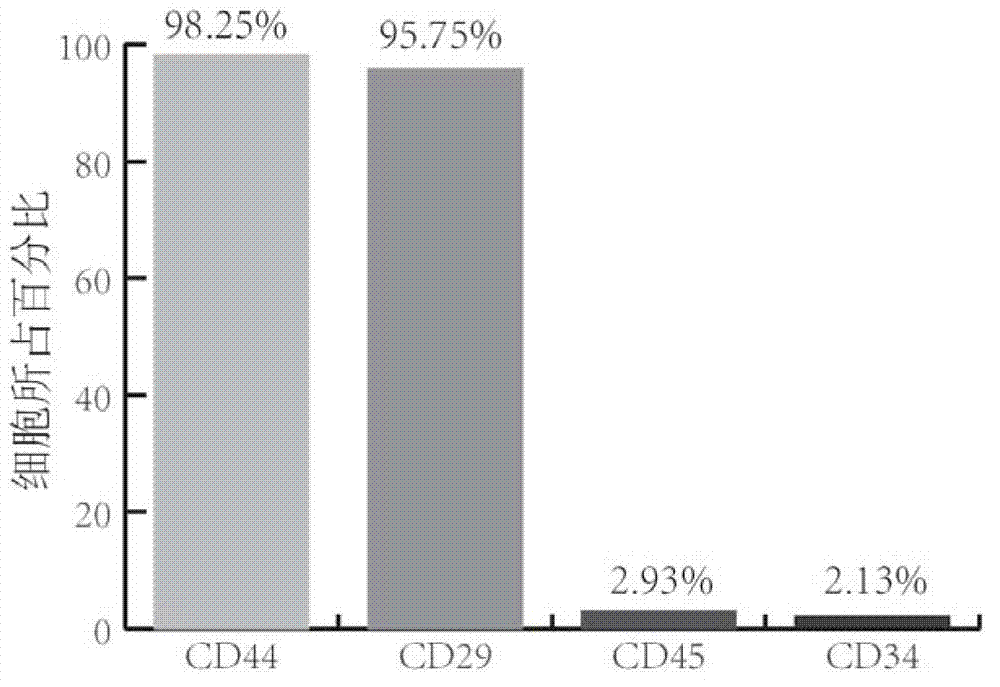
[0062] Take the detached umbilical cord tissue of full-term normal and healthy puerpera from the operating table, fully wash the residual blood with PBS balance solution, remove the umbilical vein, artery and umbilical cord adventitia, peel off Wharton glue, and cut it into 1 mm 3 For small human tissue pieces, wash the blood cells with DMEM medium repeatedly, spread the tissue pieces in a 6cm-diameter petri dish with an interval of about 1cm between the tissue pieces, and place them in 37°C, 5% CO 2 Attach to the wall for 1h in the incubator. Add 1ml of DMEM containing 10% FBS to the Petri dish, carefully place it at 37°C, 5% CO 2 cultured in an incubator. Subculture is performed after cells have climbed out of the tissue and grown into a monolayer.
[0063] Step S12. Separation and cultivation of chondrocy...
Embodiment 2
[0078] A method for inducing mesenchymal stem cells to become chondrocytes, comprising the steps of:
[0079] Step S21. Isolation of MSCs and obtaining primary cultured MSCs:
[0080] Take the umbilical cord tissue of full-term normal and healthy puerpera from the operating table, fully wash the residual blood with PBS balance solution, remove the umbilical vein, artery and umbilical cord adventitia, peel off the Wharton glue, and cut it into 1 mm 3 For small human tissue pieces, wash the blood cells with DMEM medium repeatedly, spread the tissue pieces in a 6cm-diameter petri dish with an interval of about 1cm between the tissue pieces, and place them in 37°C, 5% CO 2 Attach to the wall for 1h in the incubator. Add 1ml of DMEM containing 10% FBS to the Petri dish, carefully place it at 37°C, 5% CO 2 Cultured in an incubator, and the primary cultured MSCs were obtained after the cells climbed out of the tissue;
[0081] Step S22. Isolation of chondrocytes and obtaining prim...
Embodiment 3
[0096] A method for inducing mesenchymal stem cells to become chondrocytes, comprising the steps of:
[0097] Step S31. Isolation of MSCs and obtaining primary cultured MSCs:
[0098] Take the umbilical cord tissue of full-term normal and healthy puerpera from the operating table, fully wash the residual blood with PBS balance solution, remove the umbilical vein, artery and umbilical cord adventitia, peel off the Wharton glue, and cut it into 1 mm 3 For small human tissue pieces, wash the blood cells with DMEM medium repeatedly, spread the tissue pieces in a 6cm-diameter petri dish with an interval of about 1cm between the tissue pieces, and place them in 37°C, 5% CO 2 Attach to the wall for 1h in the incubator. Add 1ml of DMEM containing 10% FBS to the Petri dish, carefully place it at 37°C, 5% CO 2 Cultured in an incubator, and the primary cultured MSCs were obtained after the cells climbed out of the tissue;
[0099] Step S32. Isolation of chondrocytes and obtaining prim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com