Method and device for detecting carcinoembryonic antigen content
A carcinoembryonic antigen and detection method technology, which is applied in the field of biomedical analysis and detection, can solve the problems of low technical sensitivity and inability to detect early cancer, and achieve the effect of taking into account the separation effect, accurate carcinoembryonic antigen detection results, and reliable detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
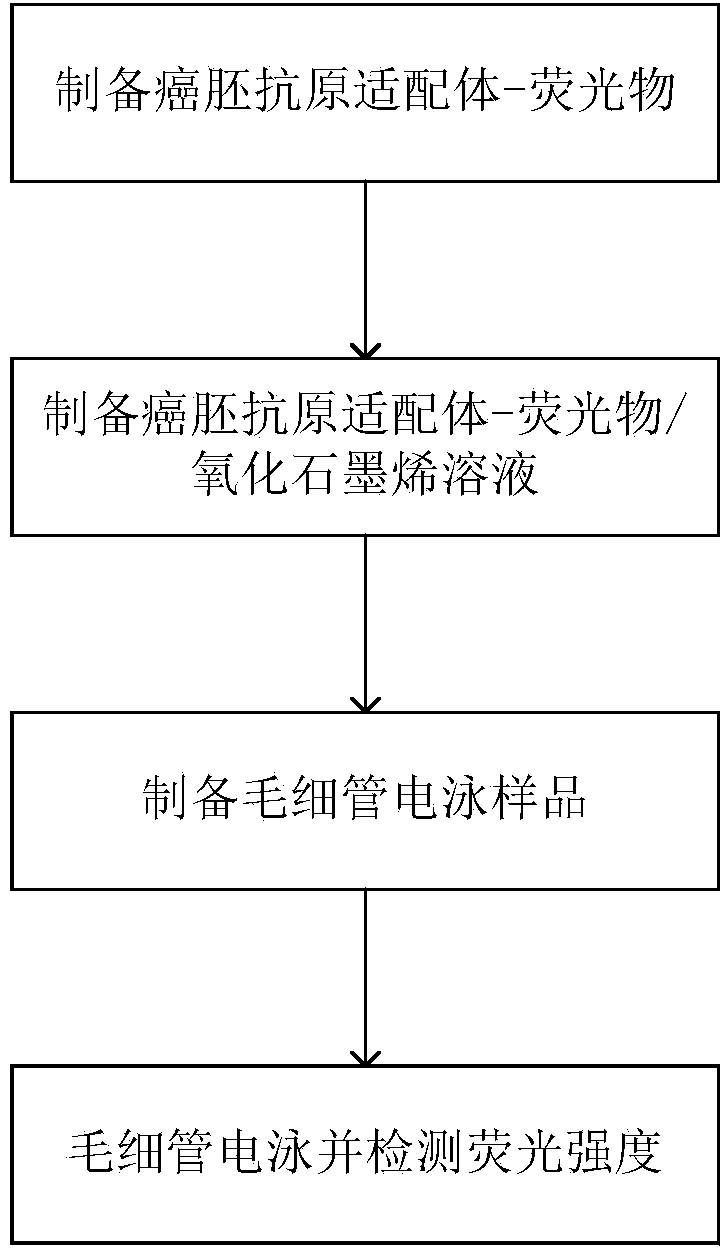
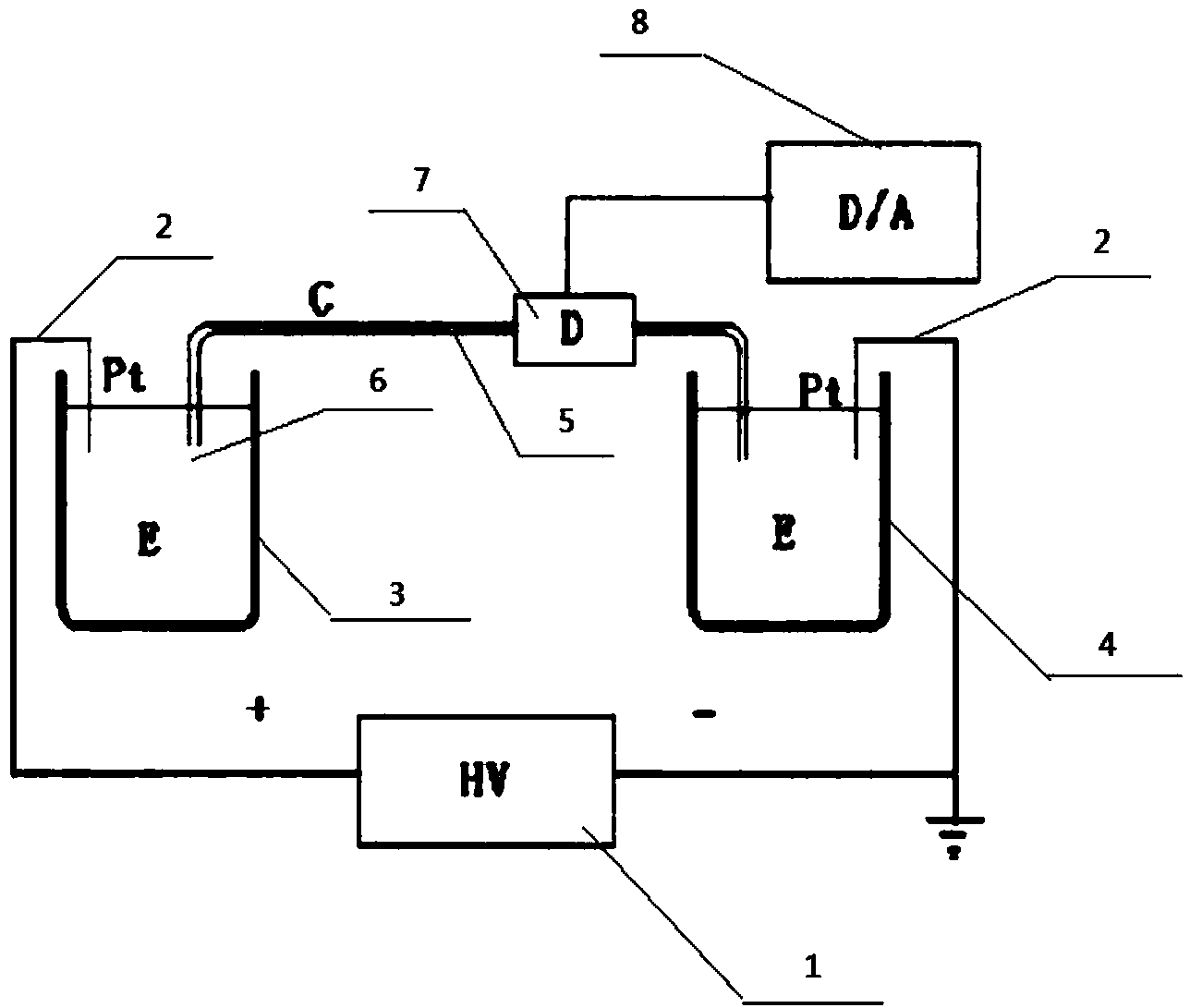
[0048] A carcinoembryonic antigen detection method, comprising the following steps:
[0049] (1) Preparation of carcinoembryonic antigen aptamer-fluorescent substance: the concentration was 10 -5 M. 30 μL of sodium thioglycolate-modified semiconductor zinc sulfide coated cadmium selenide core-shell (CdSe / ZnS) quantum dots mixed with a linker, the linker is 1-ethyl-3-(3-dimethylamino Propyl) carbodiimide hydrochloride and N-hydroxysulfosuccinimide molar ratio 5:1 mixture, this mixture is mixed with 50 μ L, 120 μ M carcinoembryonic antigen aptamer buffer solution, the buffer The solution is 0.2M phosphate buffer solution, the pH value is 8.4, so that the molar ratio of fluorescent substance, linker and carcinoembryonic antigen aptamer is 1:1000:20, so that condensation reaction occurs between fluorescent substance and aptamer After reacting for 3 hours, the mixture was centrifuged and ultrafiltered to remove excess carcinoembryonic antigen aptamer to obtain a carcinoembryonic a...
Embodiment 2
[0061] A carcinoembryonic antigen detection method, comprising the following steps:
[0062] (1) Preparation of carcinoembryonic antigen aptamer-fluorescent substance: the concentration was 10 -7 M. 30 μL bovine serum albumin-modified fluorescent nano-gold clusters are mixed with a linker, the linker is an aqueous solution of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride, and The mixture was mixed with 50μL, 6×10 -7 The carcinoembryonic antigen aptamer buffer solution of μ M is mixed evenly, and described buffer solution is the phosphate buffered saline solution of 0.2M, and pH value is 8.0, makes the molar ratio of fluorescent substance, linking agent and carcinoembryonic antigen aptamer be 1: 500:10, so that the condensation reaction between the fluorescent substance and the aptamer occurs. After 2 hours of reaction, the mixture is centrifuged and ultrafiltered to remove excess carcinoembryonic antigen aptamer, and the carcinoembryonic antigen-containing apta...
Embodiment 3
[0074] A carcinoembryonic antigen detection method, comprising the following steps:
[0075] (1) Preparation of carcinoembryonic antigen aptamer-fluorescent substance: the concentration was 10 -6 M. 30 μL of fluorescent nano-silver clusters mixed with linker, the linker is 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride and N-hydroxysulfosuccinide A 1:1 mixture of amines, the mixture was mixed evenly with 50 μL, 3 μM carcinoembryonic antigen aptamer buffer solution, the buffer solution was a 0.2M phosphate buffer solution with a pH value of 7.4, so that the fluorescent substance, the connecting The molar ratio of the reagent and the CEA aptamer is 1:200:5, so that a condensation reaction occurs between the fluorescent substance and the aptamer. After 4 hours of reaction, the mixture is centrifuged and ultrafiltered to remove excess CEA aptamer. Ligand, the obtained carcinoembryonic antigen-containing aptamer-fluorescent substance was dissolved by adding 100 μL of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com