In-situ composite system based on carbon quantum dot/manganese dioxide nanometer sheet layer and using method for detecting content of glutathione
A technology of glutathione content and carbon quantum dots, applied in the field of biological analysis, can solve the problems of high cost of gold and silver nanoclusters, unsuitable for large-scale promotion, destruction of biological samples, etc. High, adaptable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Step 1, adding carbon quantum dots to the 2-(N-morpholine) ethanesulfonic acid buffer system to prepare a carbon quantum dot solution with a concentration of 0.1 mg / ml;
[0031] Step 2. Add 5 mL of 0.2 mM potassium permanganate aqueous solution to 60 μL of the above-mentioned carbon quantum dot solution. After fully mixing, add 3 mL of deionized water to the mixed solution to prepare a reaction mixture;
[0032] Step 3: Sonicate the above reaction mixture for 20 minutes until a brown precipitate is formed. After centrifugal purification, carbon quantum dots / manganese dioxide sheet nanocomposites are obtained, and then dispersed in 8 mL of deionized water to obtain carbon quantum dots. / Manganese dioxide sheet nanocomposite system.
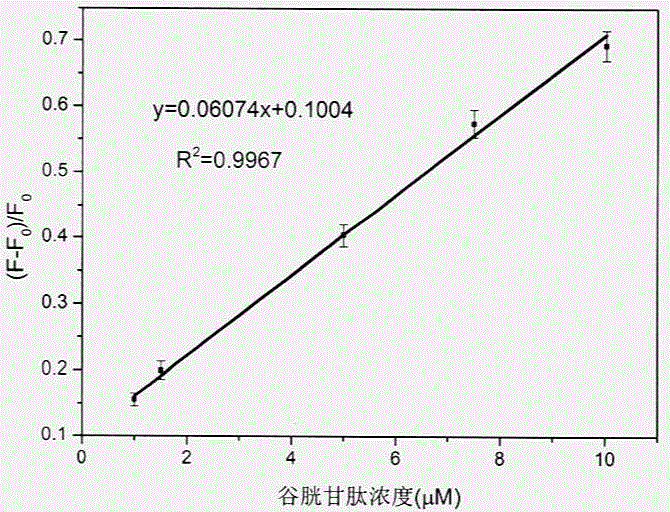
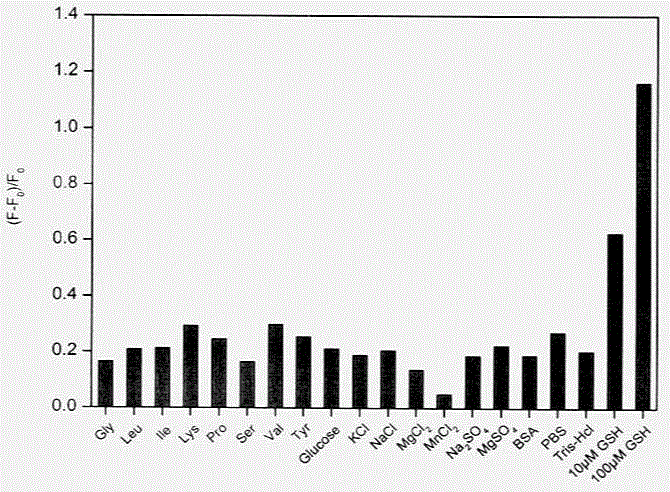
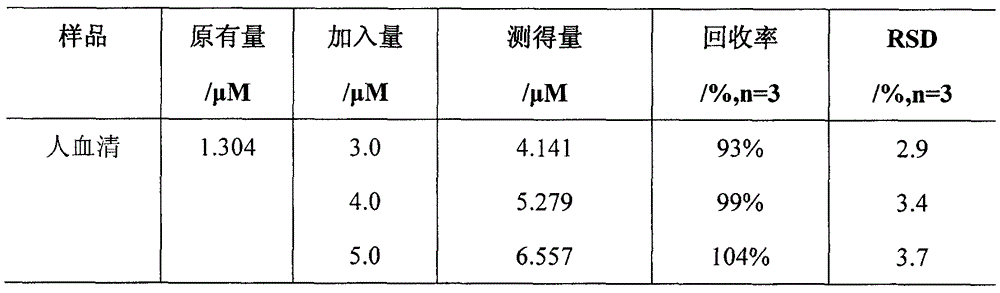
[0033] Step 4. Use the in-situ composite system based on carbon quantum dots / manganese dioxide nanosheets obtained above as a detection solution, and combine it with glutathione at concentrations of 0 μM, 1 μM, 1.5 μM, 5 μM, 7.5 μM, and 10 μ...
Embodiment 2
[0036] Step 1, adding carbon quantum dots to the 2-(N-morpholine) ethanesulfonic acid buffer system to prepare a carbon quantum dot solution with a concentration of 0.5 mg / ml;
[0037] Step 2. Add 3 mL of 0.5 mM potassium permanganate aqueous solution to 18 μL of the above-mentioned carbon quantum dot solution, and after fully mixing, add 4.5 mL of deionized water to the mixed solution to prepare a reaction mixture;
[0038] Step 3. Ultrasonic the above reaction mixture for 25 minutes until a brown precipitate is formed. After centrifugal purification, carbon quantum dots / manganese dioxide lamellar nanocomposites are obtained, and then dispersed into 12mL deionized water to obtain carbon quantum dots. / Manganese dioxide sheet nanocomposite system.
[0039] Step 4. Use the in-situ composite system based on carbon quantum dots / manganese dioxide nanosheets obtained above as a detection solution, and combine it with glutathione at concentrations of 0 μM, 1 μM, 1.5 μM, 5 μM, 7.5 μM...
Embodiment 3
[0043] Step 1, adding carbon quantum dots to the 2-(N-morpholine) ethanesulfonic acid buffer system to prepare a carbon quantum dot solution with a concentration of 1.0 mg / ml;
[0044] Step 2. Add 2 mL of potassium permanganate aqueous solution with a concentration of 1.0 mM to 12 μL of the above-mentioned carbon quantum dot solution, and after fully mixing, add 6 mL of deionized water to the mixed solution to prepare a reaction mixture;
[0045] Step 3. Ultrasonic the above reaction mixture for 35 minutes until a brown precipitate is formed. After centrifugal purification, carbon quantum dots / manganese dioxide lamellar nanocomposites are obtained, and then dispersed into 16 mL of deionized water to obtain carbon quantum dots. / Manganese dioxide sheet nanocomposite system.
[0046] Step 4. Use the in-situ composite system based on carbon quantum dots / manganese dioxide nanosheets obtained above as a detection solution, and combine it with glutathione at concentrations of 0 μM, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com