Simplified analysis method and system for pharmacokinetic parameters
A technology of pharmacokinetics and analysis methods, applied in the field of simplified analysis methods and systems of pharmacokinetic parameters, which can solve the problems of expensive WinNonlin and high cost of experimental analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
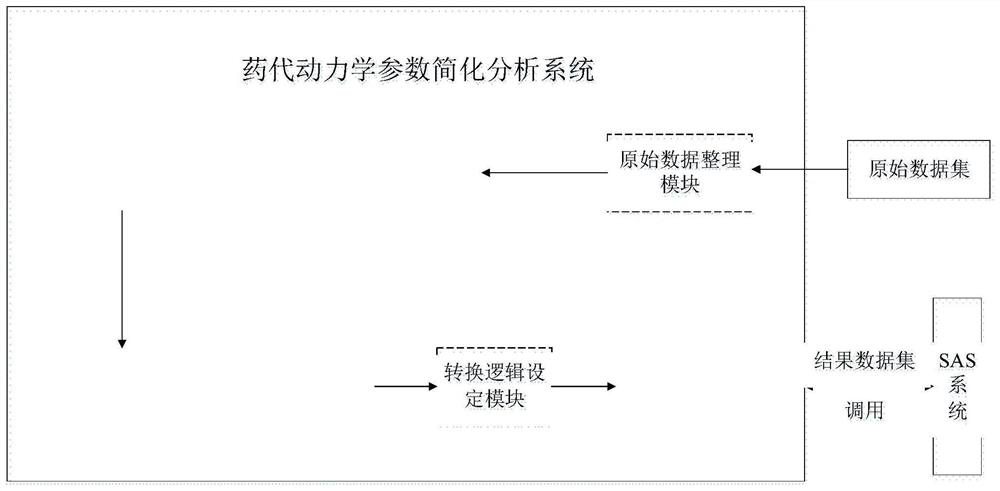
Image
Examples
Embodiment 1
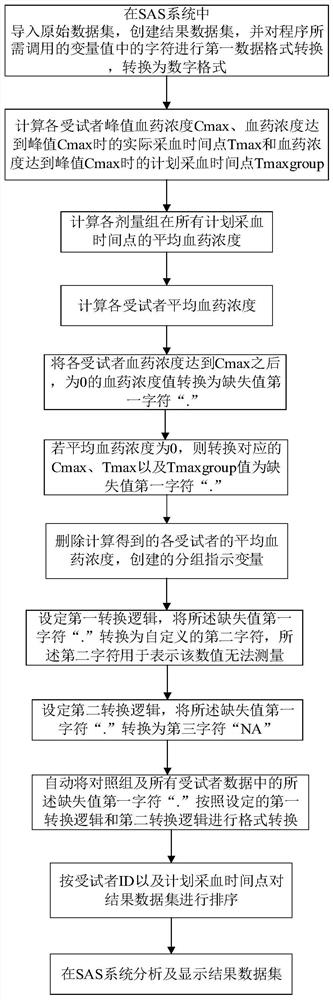
[0188] Refer to attached Figure 1-5 , according to a specific embodiment of the present invention, the simplified analysis method of pharmacokinetic parameters provided by the present invention is described in detail.
[0189] The invention provides a simplified analysis method for pharmacokinetic parameters, comprising the following steps:
[0190] S100: Import the original data set, create the result data set, and perform the first data format conversion on the characters in the variable value that the program needs to call, and convert it into a digital format;
[0191] Step S100 specifically includes the following steps:
[0192] S101: importing the original data set, extracting all the information in the original data set to create a result data set;
[0193] S102: extract and rename variables required for operation in the result data set;
[0194] S103: Convert the fourth character in the variable extracted from the result data set to the first character "." of the m...
Embodiment 2
[0265] According to a specific embodiment of the present invention, the method of the present invention implements the process of program operation, and the specific examples of defining variables include:
[0266] 1. data: is the file name of the data set to be analyzed by the program. After the user fills in the name of the data set to be analyzed by the program after data=, the program will automatically call the blood drug concentration data set according to the filled name.
[0267] 2.bql: It is the form in which the "BQL" value is marked in the original data set. Generally, the "BQL" value in the blood drug concentration data set will be directly marked as "BQL". It is marked for other forms, and the user can fill in other forms here for the program to recognize. Note that the marked form of "BQL" should be enclosed in single or double quotation marks as explicitly in the above reference code.
[0268]3.nd: It is the form that the "BQL" after reaching Cmax needs to be co...
Embodiment 3
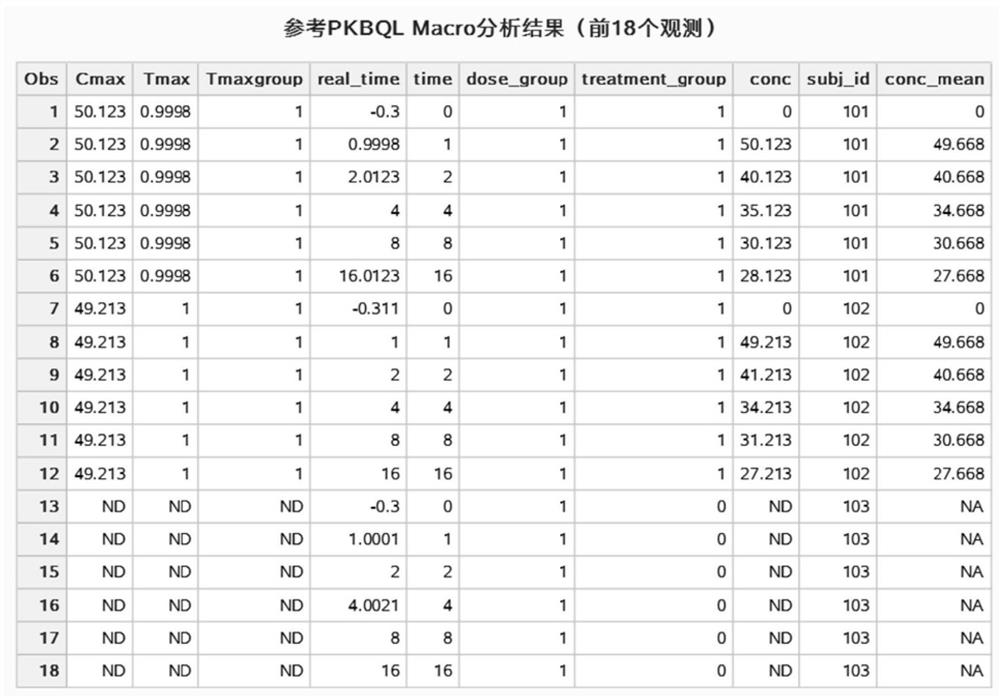
[0282] According to a specific embodiment of the present invention, taking the data in Table 1 as an example, provide the blood drug concentration of the drug clinical trial subjects at each blood collection time point before the method of the present invention is used and the data simulates a single administration. information. In the data, subj_id represents the subject number, conc represents the blood drug concentration, real_time represents the actual blood collection time point, time represents the planned blood collection time point, dose_group represents the subject’s dose group, treatment_group represents the subject’s treatment group, and 1 represents the test group, 0 represents the control group.
[0283] Table 1 Raw data set
[0284]
[0285]
[0286]
[0287]
[0288] The following is a code example of the method of the present invention called in the SAS system, and the implementation program of the method of the present invention is named "PKBQL Ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com