Pegylated exenatide ramification and use thereof
A technology of PEGylation and exenatide, which is applied in the direction of peptides, specific peptides, hormone peptides, etc., can solve the problems of protein activity loss, etc., and achieve the effects of prolonging half-life, reducing gastrointestinal emptying, and increasing the number of β cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of Pegylated Exenatide Derivatives
[0038] Exenatide compound peptides are produced by reacting covalent polyethylene glycol derivatives with thioether bonds with PEG-maleimide. Taking monomethoxypolyethylene glycol-maleimide with a molecular weight of 40,000 Daltons as an example, accurately weigh 10 mg (0.002 mmol) of Exendin-4-Cys with a length of 40 amino acids, dissolve in 5 mL of pH 7.0 50 mM phosphate buffered saline. 121 mg of polyethylene glycol maleimide (mPEG-MAL:Exendin-4-Cys molar ratio = 1.3:1) was added to the exenatide derivative solution. The reaction was carried out at room temperature for 90 min to obtain a crude Exendin-4-Cys-PEG solution. PEGylated exenatide is purified by ion exchange, electrophoresis or high performance liquid phase assay.
Embodiment 2
[0039] Example 2 Purification and determination of pegylated exenatide derivatives
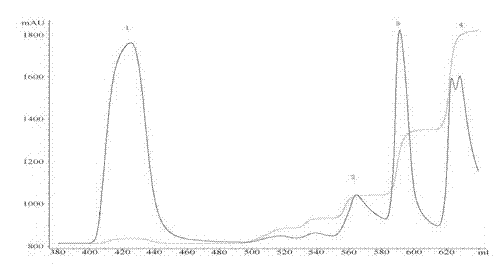
[0040] Taking the monomethoxypolyethylene glycol-maleimide-linked exenatide compound with an average molecular weight of 40,000 Daltons as an example, MacroCap SP (GE) was selected as the purification medium, and the column volume was 10 mL. 20mM NaAc buffer pH4.0 was used as buffer A, and 20mM NaAc buffer pH4.0 containing 1M NaCl was used as buffer B. Equilibrate the chromatographic column with buffer A first, and then load the sample after 5-fold dilution of buffer A. After sample loading is complete, wash 5 column volumes with Buffer A. Buffer B gradient elution, the gradient is 15%B+85%A, 25%B+75%A, 80%B+20%A, flow rate: 4mL / min, detection wavelength: 220nm detection. See the specific chromatogram figure 1 . The chromatogram shows that peak 1 is a breakthrough peak, peak 2 is an elution peak of 15%B+85%A, peak 3 is an elution peak of 25%B+75%A, and peak 4 is an elution peak of 80%B+20%...
Embodiment 3
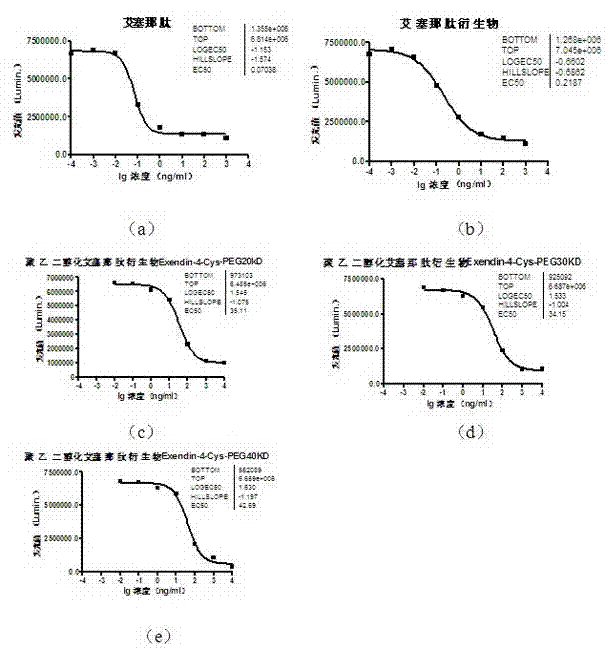
[0042] Example 3 In Vitro Biological Activity Research of Pegylated Exenatide Derivatives
[0043]Taking monomethoxypolyethylene glycol-maleimide-linked Exendin-4-Cys with an average molecular weight of 20,000, 30,000, or 40,000 Daltons as an example, the in vitro biological activity was measured using a cell reporter system. The cell line is the HEK-293 cell line (GLP-1R / HEK293) that can stably express the GLP-1 receptor. This cell line has been preserved in the General Microbiology Center of the China Committee for the Collection of Microbial Cultures, and the preservation number is CGMCC No.5909 (patent Application number 201210117317.4, title of invention: a cell line for measuring the biological activity of GLP-1 and its functional analogs and its application).
[0044] The assay process is as follows: first, the cell lines were diluted with DMEM / F12 medium containing low concentration serum, and plated in 96-well plates at 30,000 cells / 100 μL / well. 37°C, 5% CO 2 Condit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com