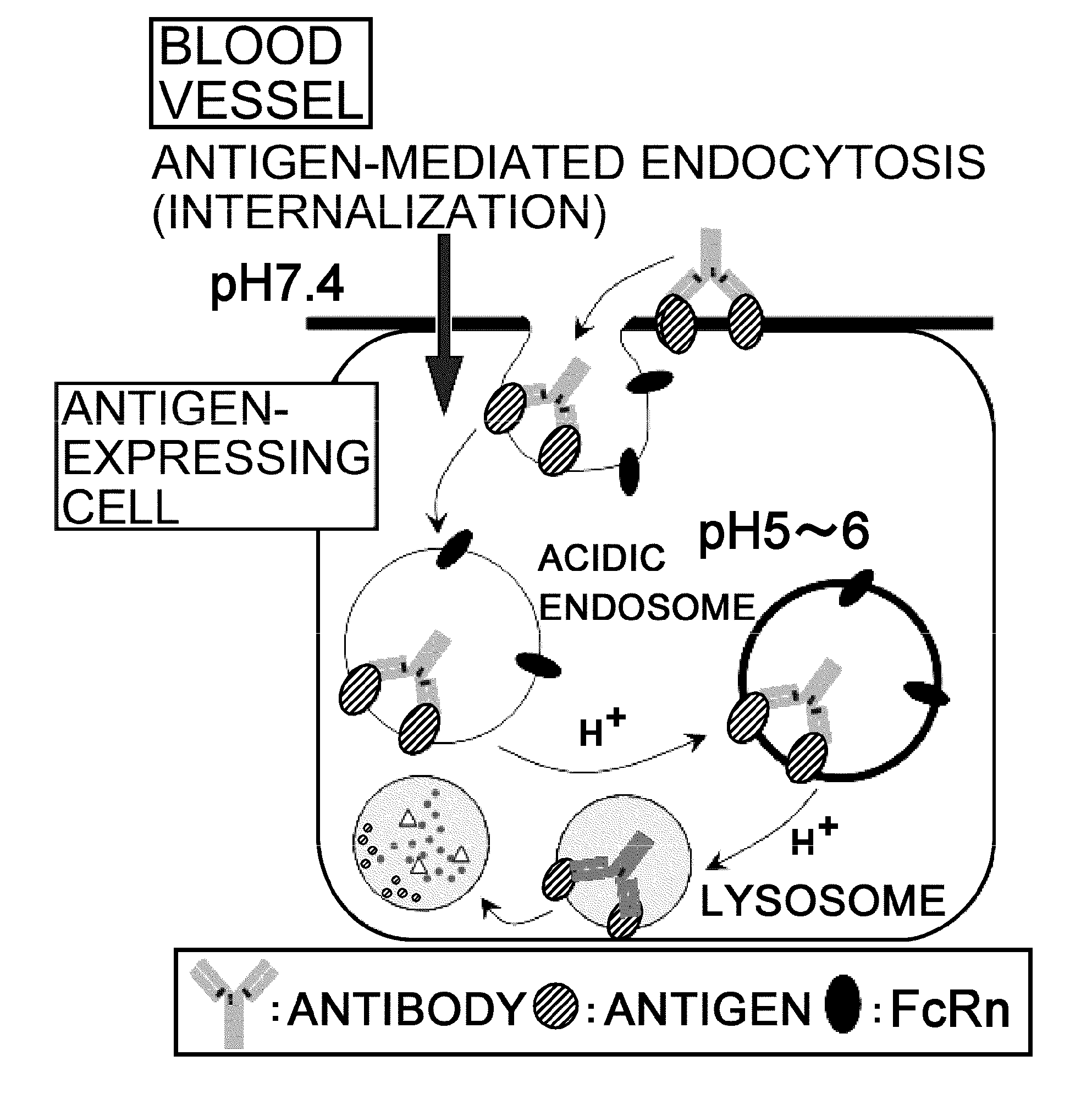
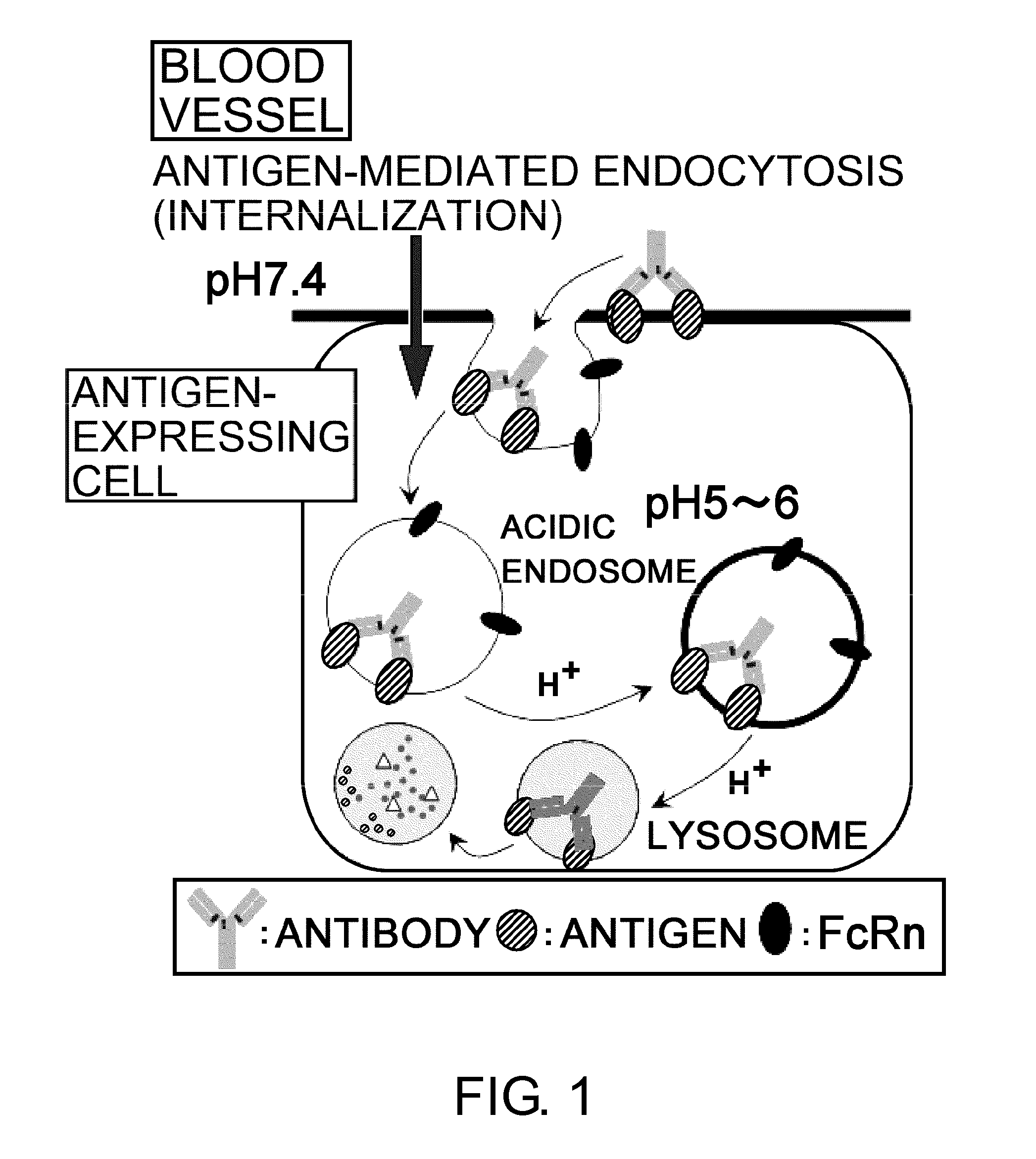
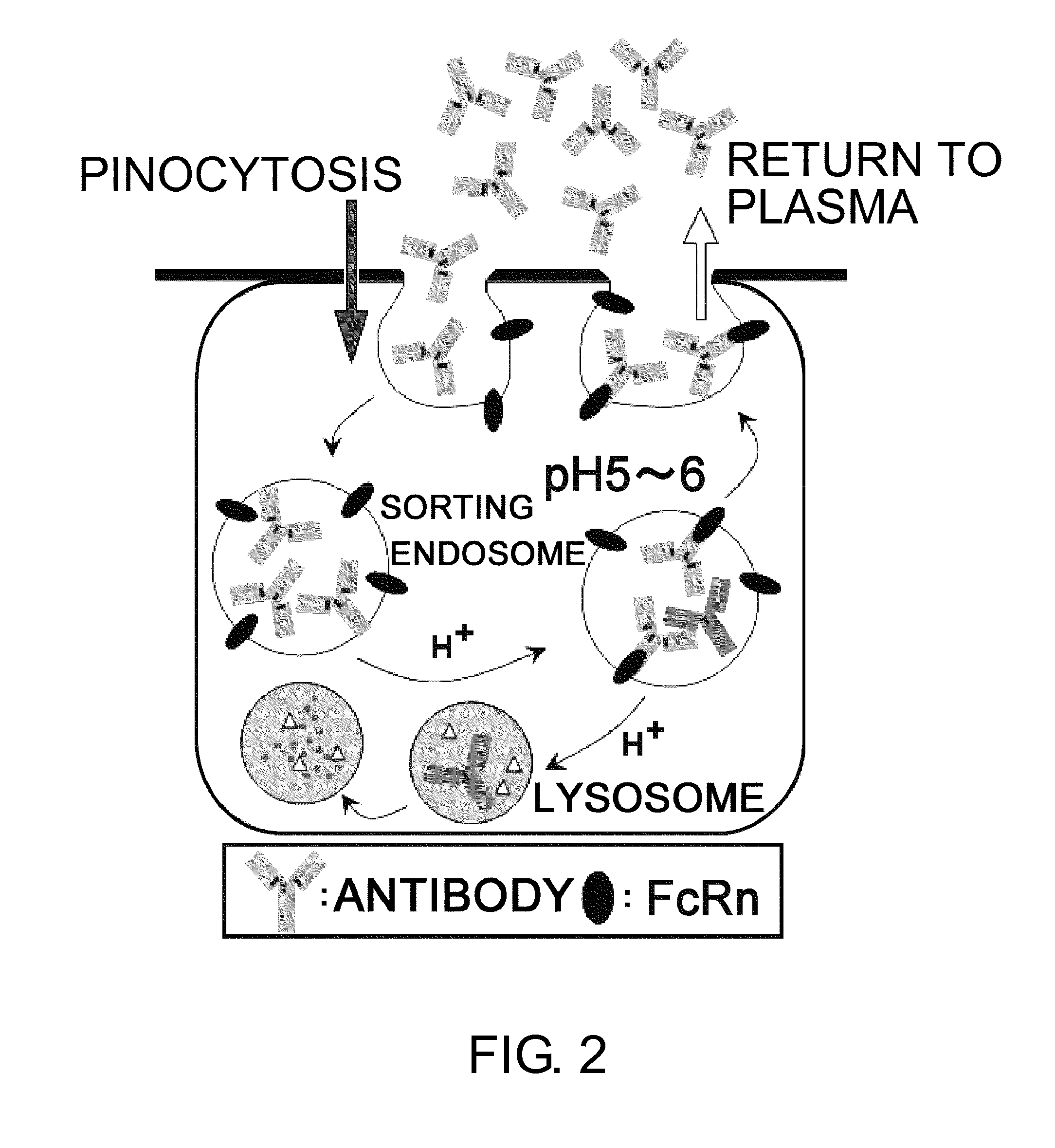
Antigen-binding molecule capable of binding to two or more antigen molecules repeatedly
a technology of antigen-binding molecules and antigen-binding peptides, which is applied in the direction of immunological disorders, drug compositions, peptides, etc., can solve the problems of high production cost, difficult subcutaneous formulation production, and inability to completely neutralize antigens with less antibodies than the amount of antigens, so as to achieve superior in vivo effects and improve the pharmacokinetics of antigen-binding molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Modified Humanized PM1 Antibody
Preparation of Recombinant Soluble Human IL-6 Receptor (SR344)
[0322]A recombinant human IL-6 receptor of the human IL-6 receptor, which served an antigen, was prepared as described below. A CHO cell line that constantly expresses a soluble human IL-6 receptor (hereinafter referred to as SR344) (Yamasaki, et al., Science 1988; 241: 825-828 (GenBank #X12830)) consisting of the amino acid sequence from the 1st amino acid to the 344th amino acid on the N-terminal side as reported in J. Biochem., 108, 673-676 (1990), was produced.
[0323]SR344 was purified from culture supernatant obtained from the SR344-expressing CHO cells using three column chromatographies: Blue Sepharose 6 FF column chromatography, affinity chromatography using a column in which an antibody specific to SR344 is immobilized, and gel filtration column chromatography. The fraction that eluted as the main peak was used as the final purified product.
Preparation of Recombinant Cy...
example 2
Production of pH-Dependently-Binding Antibody H3pI / L73
Method for Creating Antibody Capable of Neutralizing Antigen Multiple Times
[0331]Since IgG molecules are divalent, a single IgG molecule can neutralize up to two antigen molecules when the two sites bind to the antigens; however, it cannot neutralize three or more antigen molecules. Therefore, to maintain the neutralizing effect of a neutralizing antibody over a certain period, it is necessary to administer an amount of the antibody equal to or greater than the amount of antigen produced during the period. Thus, there is a limitation on the extent to which the required dose of antibody can be reduced by improving the pharmacokinetics or affinity of antibody. Therefore, if it were possible to neutralize two or more antigen molecules with a single IgG molecule, the same dose could improve the duration of neutralizing effect, or alternatively the dose of antibody required to achieve the same duration could be reduced.
[0332]For neutr...
example 3
Conferring pH-Dependent Antigen Binding Ability by His Modification of CDR Using Phage Display Technology
[0345]Production of scFv Molecule of Humanized PM1 Antibody
[0346]The humanized PM1 antibody, which is a humanized anti-IL-6R antibody (Cancer Res. 1993 Feb. 15; 53(4): 851-6), was converted into scFv. The VH and VL regions were amplified by PCR, and humanized PM1 HL scFv having the linker sequence GGGGSGGGGSGGGGS (SEQ ID NO. 15) between VH and VL was produced.
Selection of Histidine-Introducible Positions by Histidine Scanning
[0347]PCR was performed using the produced humanized PM1 HL scFv DNA as a template to produce a histidine library in which any one of the CDR amino acids is replaced with histidine. The library portions were constructed by PCR using primers in which the codon of an amino acid desired to be mutated for the library was replaced with CAT, a codon corresponding to histidine, and other portions were constructed by normal PCR. These portions were then linked by ass...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com